Gene transcriptions/TATA binding proteins/Associated factors
< Gene transcriptions < TATA binding proteins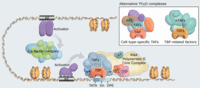
When there is no TATA box nucleotide sequence in the gene core promoter region of the DNA next to a gene, say A1BG of the human genome, a TATA binding protein associated factor (TAF) will bind sequence specifically and force the TATA box binding protein to bind non-sequence specifically to the DNA in the core promoter.
Notations
Notation: let the symbol TAF stand for TATA binding protein associated factor.
TATA boxes
A TATA box is a common type of core promoter sequence in eukaryotes which is a short DNA sequence.
TATA binding proteins
"The TATA-binding protein (TBP) is a general transcription factor that binds specifically to a DNA sequence called the TATA box."[1]
Initiator elements
Notation: let the symbol Inr denote an initiator element.
Notation: let the symbol +1 designate the nucleotide that is the transcription start site (TSS).
Most human genes lack a TATA box and use an Inr or downstream promoter element instead. As in other metazoans, for genes lacking a TATA box, the Inr is functionally analogous, with a base pair (bp) consensus 5'-YYA+1NWYY-3', to direct transcription initiation.[2] On the template strand (used as a template for RNA synthesis), the consensus sequence is 3'-YYA+1NWYY-5'.
The Inr is the only element in metazoan protein-encoding genes known to be a functional analog of the TATA box, in that it is sufficient for directing accurate transcription initiation in genes that lack TATA boxes.[3] An Inr for mammalian RNA polymerase II can be defined as a DNA sequence element that overlaps a TSS and is sufficient for
- determining the start site location in a promoter that lacks a TATA box and
- enhancing the strength of a promoter that contains a TATA box.[4]
"Although any isolated TAF may not exhibit sequence-specific interactions at the Inr element in the absence of a TATA-box, a combination of TAFs may bind sequence specifically to the Inr element regardless of the TATA-box and/or DPE (Chalkley and Verrijzer, 1999)."[5] Bold added.
TAF1
TAF1 "binds to core promoter sequences encompassing the transcription start site. It also binds to activators and other transcriptional regulators, and these interactions affect the rate of transcription initiation."[6] TAF1 "is part of a complex transcriptional unit (TAF1/DYT3)"[6].
"Yeast TAF1 can be divided into four regions including a putative histone acetyltransferase domain and TBP, TAF, and promoter binding domains."[7]
"TAF1 [has] been systematically dissected into ... functional domains: an N-terminal TBP-binding domain termed TAND, a TAF-TAF interaction domain, a putative histone acetyltransferase (HAT) domain, ... a promoter recognition domain [(PB1), and] a ... domain that interacts with TAF7"[7]. The promoter recognition domain is approximately at one end of the gene for TAF1.[7]
On human chromosome X (number 23, NC_000023), specifically NC_000023.10, TAF1 is located 3'-70586113[-70685855]-5', 99,742 nt.[8] The 3'-UTR begins at 70586114.[8]
TAF1 isoform 1
Isoform 1 (variant 1) "represents the longer transcript and encodes the longer isoform"[6].
TAF1 isoform 2
Isoform 2 (variant 2) "uses an alternate in-frame splice site, compared to variant 1, resulting in a shorter isoform (2) that lacks an internal 21 aa segment, compared to isoform 1."[6]
TAF1/DYT3
TAF1/DYT3 is "a complex transcript system that is composed of at least 43 exons. Thirty-eight exons code for [TAF1] ... Five downstream exons (d1-d5) ... can either form transcripts with TAF1 exons or be transcribed independently."[9] "Transcripts including exons d1, d3, d4, d5, plus TAF1 exons make up transcript "variant 1." Major "variant 2" is composed of various TAF1 exons plus exons d3 and d4. Alternately, d exons can generate transcripts independent of TAF1 exons 1-38. Exons d2, d3, and d4 make up "variant 3" and exons d3 and d4 constitute "variant 4.""[9] The "additional five exons are located 3' to exon 38 ... ("downstream" exons 1-5)"[9].
TAF1/TAF2
"[A] [TAF1]-[TAF2] complex selects sequences that match the Initiator (Inr) consensus."[10]
TAF1L
TAF1L "is expressed in male germ cells, and the product has been shown to function interchangeably with the TAF1 product."[11]
TAF2
TAF2 "is stably associated with the TFIID complex. It contributes to interactions at and downstream of the transcription initiation site, interactions that help determine transcription complex response to activators."[12]
TAF3
TAF3 is part of the "set of TBP-associated factors (TAFs) [which] contribute to promoter recognition and selectivity and act as antiapoptotic factors"[13]
TAF4
TAF4 "has been shown to potentiate transcriptional activation by retinoic acid, thyroid hormone and vitamin D3 receptors. In addition, [it] interacts with the transcription factor CREB, which has a glutamine-rich activation domain, and binds to other proteins containing glutamine-rich regions."[14]
TAF4B
TAF4B is "a cell type-specific TAF that may be responsible for mediating transcription by a subset of activators in B cells."[15]
TAF5
TAF5 is "an integral subunit of TFIID associated with all transcriptionally competent forms of that complex. [It] interacts strongly with two TFIID subunits that show similarity to histones H3 and H4, and it may participate in forming a nucleosome-like core in the TFIID complex."[16]
TAF6
TAF6 "binds weakly to TBP but strongly to TAF1"[17].
TAF7
TAF7 "interacts with the largest TFIID subunit, as well as multiple transcription activators. [It] is required for transcription by promoters targeted by RNA polymerase II."[18]
TAF7L
TAF7L "could be a spermatogenesis-specific component of the DNA-binding general transcription factor complex TFIID."[19]
TAF8
TAF8 "contains an H4-like histone fold domain, and interacts with several subunits of TFIID including TBP and the histone-fold protein TAF10."[20]
TAF9
TAF9 "binds to the basal transcription factor GTF2B as well as to several transcriptional activators such as p53 and VP16."[21]
TAF10
TAF10 "is associated with a subset of TFIID complexes. Studies with human and mammalian cells have shown that this subunit is required for transcriptional activation by the estrogen receptor, for progression through the cell cycle, and may also be required for certain cellular differentiation programs."[22]
TAF11
TAF11 "is present in all TFIID complexes and interacts with TBP. This subunit also interacts with another small subunit, TAF13, to form a heterodimer with a structure similar to the histone core structure."[23]
TAF12
"TAF12 interacts directly with TBP as well as with TAF2I [(TAF11)]."[24]
TAF13
TAF13 "interacts with TBP and with two other small subunits of TFIID, TAF10 and TAF11."[25]
TAF15
TAF15 is in "a subunit of TFIID present in a subset of TFIID complexes. Translocations involving chromosome 17 and chromosome 9, where the gene for the nuclear receptor CSMF is located, result in a gene fusion product that is an RNA binding protein associated with a subset of extraskeletal myxoid chondrosarcomas."[26]
General transcription factor II D
"Before the start of transcription, the transcription factor II D (TFIID) complex, binds to the ... core promoter of the gene."[27]
Research
Hypothesis:
- TAFs are not involved in the transcription of A1BG.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[28] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[29]"[30]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[31] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[32]
See also
References
- ↑ "TATA-binding protein, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 30, 2012. Retrieved 2012-09-29.
- ↑ DR Liston, PJ Johnson (March 1999). "Analysis of a Ubiquitous Promoter Element in a Primitive Eukaryote: Early Evolution of the Initiator Element". Molecular and Cellular Biology 19 (3): 2380-8. PMID 10022924.
- ↑ ST Smale (March 1997). "Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes". Biochimica & Biophysica Acta 1351 (1-2): 73-88. doi:10.1016/S0167-4781(96)00206-0. PMID 9116046.
- ↑ R. Javahery, A. Khachi, K. Lo, B. Zenzie-Gregory, S. T. Smale (January 1994). "DNA Sequence Requirements for Transcriptional Initiator Activity in Mammalian Cells". Molecular and Cellular Biology 14 (1): 116-27. PMID 8264580.
- ↑ Ananda L. Roy (August 2001). "Biochemistry and biology of the inducible multifunctional transcription factor TFII-I". Gene 274 (1-2): 1-13. doi:10.1016/S0378-1119(01)00625-4. http://www.sciencedirect.com/science/article/pii/S0378111901006254. Retrieved 2012-04-06.
- 1 2 3 4 HGNC:11535 (March 24, 2012). "TAF1 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 250kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- 1 2 3 Jordan D. Irvin and B. Franklin Pugh (March 10, 2006). "Genome-wide Transcriptional Dependence on TAF1 Functional Domains". The Journal of Biological Chemistry 281 (10): 6404-12. doi:10.1074/jbc.M513776200. PMID 16407318. http://www.ncbi.nlm.nih.gov/pubmed/16407318. Retrieved 2012-04-14.
- 1 2 Ncbi. "Homo sapiens chromosome X, GRCh37.p5 Primary Assembly". Rockville, MD: National Center for Biotechnology Information, US National Library of Medicine. Retrieved 2012-04-25.
- 1 2 3 Thilo Herzfeld, Dagmar Nolte and Ulrich Müller (2007). "Structural and functional analysis of the human TAF1/DYT3 multiple transcript system". Mammalian Genome 18 (11): 787-95. doi:10.1007/s00335-007-9063-z. http://www.springerlink.com/content/f75q704142277344/. Retrieved 2012-04-25.
- ↑ Gillian E. Chalkley and C. Peter Verrijzer (September 1, 1999). "DNA binding site selection by RNA polymerase II TAFs: a TAFII250-TAFII150 complex recognizes the Initiator". The EMBO Journal 18 (17): 4835-45. PMID 10469661. http://www.ncbi.nlm.nih.gov/pubmed/10469661. Retrieved 2012-04-26.
- ↑ HGNC:18056 (March 10, 2012). "TAF1L RNA polymerase II, TATA box binding protein (TBP)-associated factor, 210kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11536 (March 10, 2012). "TAF2 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 150kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:17303 (March 24, 2012). "TAF3 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 140kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11537 (March 10, 2012). "TAF4 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 135kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11538 (March 17, 2012). "TAF4b RNA polymerase II, TATA box binding protein (TBP)-associated factor, 105kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11539 (March 10, 2012). "TAF5 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 100kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11540 (March 24, 2012). "TAF6 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 80kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11541 (March 10, 2012). "TAF7 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 55kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11548 (March 10, 2012). "TAF7-like RNA polymerase II, TATA box binding protein (TBP)-associated factor, 50kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:17300 (March 10, 2012). "TAF8 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 43kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11542 (March 31, 2012). "TAF9 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 32kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11543 (March 24, 2012). "TAF10 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 30kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11544 (March 10, 2012). "TAF11 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 28kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11545 (March 17, 2012). "TAF12 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 20kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11546 (March 24, 2012). "TAF13 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 20kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ HGNC:11547 (March 17, 2012). "TAF15 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 68kDa". Bethesda, Maryland: NCBI. Retrieved 2012-04-09.
- ↑ "Transcription factor II D, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 7, 2012. Retrieved 2012-09-30.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
Further reading
- Ananda L. Roy (August 2001). "Biochemistry and biology of the inducible multifunctional transcription factor TFII-I". Gene 274 (1-2): 1-13. doi:10.1016/S0378-1119(01)00625-4. http://www.sciencedirect.com/science/article/pii/S0378111901006254. Retrieved 2012-04-06.
External links
- African Journals Online
- Bing Advanced search
- GenomeNet KEGG database
- Google Books
- Google scholar Advanced Scholar Search
- Home - Gene - NCBI
- JSTOR
- Lycos search
- NASA's National Space Science Data Center
- NCBI All Databases Search
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- Questia - The Online Library of Books and Journals
- SAGE journals online
- Scirus for scientific information only advanced search
- SpringerLink
- Taylor & Francis Online
- WikiDoc The Living Textbook of Medicine
- Wiley Online Library Advanced Search
- Yahoo Advanced Web Search
| |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a biochemistry resource. |
| |
Subject classification: this is a medicine resource. |