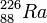Radioactivity
Radioactivity
The nuclei of some atoms are spontaneously disintegrate from one form of isotope to another. until they reach stable form. These atoms emitparticles (alpha, beta, gamma) which are different in charge, size, penetrating power and ionization energy.
Half life of the isotope
The half - life of isotope is the time, needed to decay the isotope so that half of it's mass is remaining. Here is an example. Lets say we have 50 grams of the radioactive isotope Carbon 14, which has a half-life of 5,730 years. After 5,730 years, 25 grams will remain. After another 5,730 years have passed only 12.5 grams remain and so on, until the parent isotope has completely decayed into a daughter isotope, in which either it will decay into another isotope, or remain the same, depending if it is stable or radioactive.
Types of decay
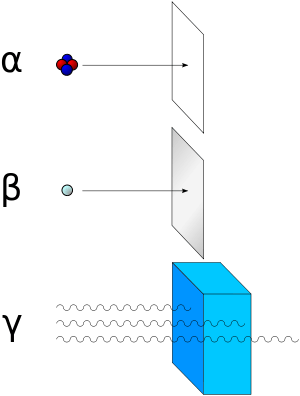
There are three main types of radioactive decay; these include Alpha, Beta, & Gamma Radiation. Alpha and Beta decay are released in particles, while Gamma radiation is released in rays.[1]
Gamma radiation is much stronger than the other two types, as an alpha particle can be stopped by a thin piece of paper, and a beta particle can be stopped by a sheet of aluminum foil, gamma can penetrate both with ease. The most efficient way to stop gamma rays, are through the element lead (You may have wondered why you wear a lead bib or covering when you get a dental X-Ray).
Here are some common radiation emitters:
- Alpha
Americium-241
Radon
- Beta
Strontium-90
- Gamma
Uranium-238
References
- ↑ Though, because of the wave-particle duality of Quantum mechanics, gamma rays can also be thought of as particles.
Example of alpha decay
See also
- Atomic Structure
- Age of the Earth - practical application of radioactive decay