Radiation astronomy/Colors
< Radiation astronomy
Each form of radiation that an astronomical entity can emit, absorb, fluoresce, transmit, or reflect has a spectrum in time, space, intensity, or variation.
A spectrum of variation shows its colors.
Astronomy
Astronomy in general consists of observing the types of spectra apparently originating with an astronomical entity and describing these observations empirically if necessary with theory.
Radiation
Radiation in its spectrum of forms from meteors to Superluminals shows variation, time, space, and intensity.
Minerals




"Ultraviolet lamps are also used in analyzing minerals and gems, ... Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light, or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet."[1]
"Ultraviolet lamps may cause certain minerals to fluoresce, and is a key tool in prospecting for tungsten mineralisation."[2]
"Many samples of fluorite exhibit fluorescence under ultraviolet light, a property that takes its name from fluorite.[3] Many minerals, as well as other substances, fluoresce. Fluorescence involves the elevation of electron energy levels by quanta of ultraviolet light, followed by the progressive falling back of the electrons into their previous energy state, releasing quanta of visible light in the process. In fluorite, the visible light emitted is most commonly blue, but red, purple, yellow, green and white also occur. The fluorescence of fluorite may be due to mineral impurities such as yttrium, ytterbium, or organic matter in the crystal lattice. In particular, the blue fluorescence seen in fluorites from certain parts of Great Britain responsible for the naming of the phenomenon of fluorescence itself, has been attributed to the presence of inclusions of divalent europium in the crystal.[4]"[5]
"Between 190 and 1700 nm, the ordinary refractive index varies roughly between 1.9 and 1.5, while the extraordinary refractive index varies between 1.6 and 1.4.[6]"[7]
"Under longwave (365 nm) ultraviolet light, diamond may fluoresce a blue, yellow, green, mauve, or red of varying intensity. The most common fluorescence is blue, and such stones may also phosphoresce yellow—this is thought to be a unique combination among gemstones. There is usually little if any response to shortwave ultraviolet".[8]
Meteors










A spectrum of meteors may range in size (space), speed (time), numbers (intensity), or state (variation, e.g. rocky, liquid, gaseous, or plasma).
"The 1910 approach, which came into naked-eye view around 10 April[9] and came to perihelion on 20 April,[9] was notable for several reasons: it was the first approach of which photographs exist, and the first for which spectroscopic data were obtained.[10] Furthermore, the comet made a relatively close approach of 0.15AU,[9] making it a spectacular sight. Indeed, on 19 May, the Earth actually passed through the tail of the comet.[11][12] One of the substances discovered in the tail by spectroscopic analysis was the toxic gas cyanogen,[13] which led astronomer Camille Flammarion to claim that, when Earth passed through the tail, the gas "would impregnate the atmosphere and possibly snuff out all life on the planet."[14] His pronouncement led to panicked buying of gas masks and quack "anti-comet pills" and "anti-comet umbrellas" by the public.[15] In reality, as other astronomers were quick to point out, the gas is so diffuse that the world suffered no ill effects from the passage through the tail.[14]"[16]
"It is quite possible that [faint streamers preceding the main tail and lying nearly in the prolonged radius vector] may have touched the Earth, probably between May 19.0 and May 19.5, [1910,] but the Earth must have passed considerably to the south of the main portion of the tail [of Halley's comet]."[17]
"A typical comet nucleus has an albedo of 0.04.[18]"[19]
At left is an image of Comet West. "Comet West was a stunning sight in the predawn sky of March, 1976, bright with a tall and broad dust tail. ... [T]he comet [was] discovered on photographs taken in August 1975 by Richard West of the European Southern Observatory ... Comet West passed perihelion on February 25, 1976, at a distance of 0.20 a.u. [and] had reached about magnitude -3 at perihelion. Several observers saw it telescopically in daylight, and John Bortle observed it with the naked eye shortly before sunset. ... The following morning, March 7, ... It was brilliant, with a head as bright as Vega (which was nearly overhead) and a huge tail, about 20 degrees tall, straight near the bottom and bending to the left in its upper reaches. The comet quickly faded during March".[20]
Although many comets are photographed in black and white, not that many are actually only white but have colors. The image at right of McNaught Comet shows white and other colors, as does Comet West at left.
Of the Local Group, “[i]ts two dominant galaxies, the Milky Way and Andromeda (M31), are separated by a distance of ~700 kpc and are moving toward each other with a radial velocity of about -117 km s-1 (Binney & Tremaine 1987, p. 605).”[21] "making [Andromeda] one of the few blueshifted galaxies. The Andromeda Galaxy and the Milky Way are thus expected to collide in about 4.5 billion years, although the details are uncertain since Andromeda's tangential velocity with respect to the Milky Way is only known to within about a factor of two.[22] A likely outcome of the collision is that the galaxies will merge to form a giant elliptical galaxy.[23] Such events are frequent among the galaxies in galaxy groups. The fate of the Earth and the Solar System in the event of a collision are currently unknown. If the galaxies do not merge, there is a small chance that the Solar System could be ejected from the Milky Way or join Andromeda.[24]"[25]
Cosmic rays
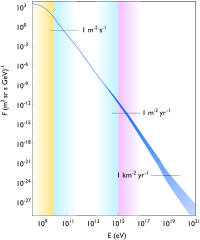
At right is an image indicating the range of cosmic-ray energies. The flux for the lowest energies (yellow zone) is mainly attributed to solar cosmic rays, intermediate energies (blue) to galactic cosmic rays, and highest energies (purple) to extragalactic cosmic rays.[26]
Cosmic rays may be upwards of a ZeV (1021 eV).
“About 89% of cosmic rays are simple protons or hydrogen nuclei, 10% are helium nuclei of alpha particles, and 1% are the nuclei of heavier elements. ... Solitary electrons ... constitute much of the remaining 1%.”[27]
Neutrons
"The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adopted to the Maxwellian distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy is of the free neutron. Kinetic energy, speed and wavelength of the neutron are related through the De Broglie relation."[28]
"Moderated and other, non-thermal neutron energy distributions or ranges are ...
- Fast neutrons [with] kinetic energies greater than 1 eV, 0.1 MeV or approximately 1 MeV, depending on the definition.
- Slow neutrons ... a kinetic energy less than or equal to 0.4 eV.
- Epithermal neutrons ... an energy from 1 eV to 10 keV.
- Hot neutrons ... an energy of about 0.2 eV.
- Thermal neutrons ... an energy of about 0.025 eV.[29] This is the most probable energy, while the average energy is 0.038 eV.
- Cold neutrons ... an energy from 5 × 10−5 eV to 0.025 eV.
- Very cold neutrons ... an energy from 3 × 10−7 eV to 5 × 10−5 eV.
- Ultra cold neutrons ... an energy less than 3 × 10−7 eV.
- Continuum region neutrons ... an energy from 0.01 MeV to 25 MeV.
- Resonance region neutrons ... an energy from 1 eV to 0.01 MeV.
- Low energy region neutrons ... an energy less than 1 eV."[28]
Electrons
Notation: WN5 is a component of V444 Cygni, with its Wolf-Rayet (W) spectrum dominated by NitrogenIII-V and HeliumI-II lines and WN2 to WN5 considered hotter or "early".
"The color temperature of the central part of the WN5 disk for λ < 7512 Å, where the main source of opacity is electron scattering, is Tc = 80,000-100,000 K. This high temperature represents the electron temperature slightly below the surface of the WN5 core--the level at which the star becomes optically thick in electron scattering."[30]
Muons
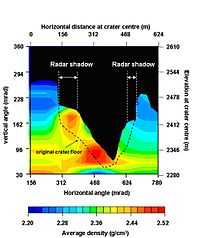
The colors chosen for the image on the right relate to density of the rock.
Neutrinos
“Neutrino oscillation is a quantum mechanical phenomenon predicted by Bruno Pontecorvo[31] whereby a neutrino created with a specific lepton flavor (electron, muon or tau) can later be measured to have a different flavor. The probability of measuring a particular flavor for a neutrino varies periodically as it propagates. Neutrino oscillation is of theoretical and experimental interest since observation of the phenomenon implies that the neutrino has a non-zero mass”.[32]
Opticals
| Class | B–V | U–B | V–R | R–I | Teff (K) |
|---|---|---|---|---|---|
| O5V | –0.33 | –1.19 | –0.15 | –0.32 | 42,000 |
| B0V | –0.30 | –1.08 | –0.13 | –0.29 | 30,000 |
| A0V | –0.02 | –0.02 | 0.02 | –0.02 | 9,790 |
| F0V | 0.30 | 0.03 | 0.30 | 0.17 | 7,300 |
| G0V | 0.58 | 0.06 | 0.50 | 0.31 | 5,940 |
| K0V | 0.81 | 0.45 | 0.64 | 0.42 | 5,150 |
| M0V | 1.40 | 1.22 | 1.28 | 0.91 | 3,840 |
"[T]he color index is a simple numerical expression that determines the color of an object, which in the case of a star gives its temperature. To measure the index, one observes the magnitude of an object successively through two different filters, such as U and B, or B and V, where U is sensitive to ultraviolet rays, B is sensitive to blue light, and V is sensitive to visible (green-yellow) light (see also: UBV system). The set of passbands or filters is called a photometric system. The difference in magnitudes found with these filters is called the U-B or B–V color index, respectively. The smaller the color index, the more blue (or hotter) the object is. Conversely, the larger the color index, the more red (or cooler) the object is. This is a consequence of the logarithmic magnitude scale, in which brighter objects have smaller (more negative) magnitudes than dimmer ones. For comparison, the yellowish Sun has a B–V index of 0.656 ± 0.005,[34] while the bluish Rigel has B–V –0.03 (its B magnitude is 0.09 and its V magnitude is 0.12, B–V = –0.03).[35] The passbands most optical astronomers use are the UBVRI filters, where the U, B, and V filters are as mentioned above, the R filter passes red light, and the I filter passes infrared light. ... These filters were specified as particular combinations of glass filters and photomultiplier tubes."[36]
"[A] Photometric system is a set of well-defined passbands (or filters), with a known sensitivity to incident radiation. The sensitivity usually depends on the optical system, detectors and filters used. For each photometric system a set of primary standard stars is provided."[37]
| Filter Letter | Effective Wavelength Midpoint λeff For Standard Filter[38] | Full Width Half Maximum[38] | Variant(s) | Description |
|---|---|---|---|---|
| Ultraviolet | ||||
| U | 365nm | 66nm | u, u', u* | "U" stands for ultraviolet. |
| Visible | ||||
| B | 445nm | 94nm | b | "B" stands for blue. |
| V | 551nm | 88nm | v, v' | "V" stands for visual. |
| G | g, g' | "G" stands for green (visual). | ||
| R | 658nm | 138nm | r, r', R', Rc, Re, Rj | "R" stands for red. |
| Near-Infrared | ||||
| I | 806nm | 149nm | i, i', Ic, Ie, Ij | "I" stands for infrared. |
Visuals
| ||||||||

"Grey or gray is an intermediate color between black and white, a neutral or achromatic color, meaning literally a color "without color." [39] It is the color of a cloud-covered sky, of ash and of lead.[40]"[41]
The first image at right shows some red pebbles among gray pebbles, which are all the same rock type.
The first image at left shows the various shades of grey.
| ||||||||

"White is the color of fresh milk and snow.[42][43] It is the color the human eye sees when it looks at light which contains all the wavelengths of the visible spectrum, at full brightness and without absorption. It does not have any hue.[44]"[45]
"de la couleur de la neige, du lait. Lumiere resultant de la combinaison de toutes les couleurs du spectre solaire."[46] (of the color of snow, of milk. Light resulting from the combination of all the colors of the solar spectrum.)
The white cliffs shown in the image at right are made of chalk, or calcium carbonate.
"[W]hite light reflected off objects can be seen when no part of the light spectrum is reflected significantly more than any other and the reflecting material has a degree of diffusion. People see this when transparent fibers, particles, or droplets are in a transparent matrix of a substantially different refractive index. Examples include classic "white" substances such as sugar, foam, pure sand or snow, cotton, clouds, and milk. Crystal boundaries and imperfections can also make otherwise transparent materials white, as in the milky quartz or the microcrystalline structure of a seashell."[45]
"Cumulus clouds look white because the water droplets reflect and scatter the sunlight without absorbing other colors."[45]
"White light is generated by the sun, by stars, or by earthbound sources such as incandescent lamps, fluorescent lamps and white LEDs."[45]
| |||||


"Black is the color of coal, ebony, and of outer space. It is the darkest color, the result of the absence of or complete absorption of light. It is the opposite of white and often represents darkness in contrast with light.[47][48][49]"[50]
"Opposite to white: colourless from the absence or complete absorption of light. Also, so near this as to have no distinguishable colour, very dark."[47]
Black is "[t]he darkest color".[48]
"Se dit de la couleur la plus foncée, due à l'absence ou à l'absorption totale des rayons lumineux."[49]("said of the very darkest color, due to the absence or complete absorption of all rays of light.")
| | ||
|---|---|---|
| Color | Frequency | Wavelength |
| violet | 668–789 THz | 380–450 nm |
| blue | 631–668 THz | 450–475 nm |
| cyan | 606–630 THz | 476–495 nm |
| green | 526–606 THz | 495–570 nm |
| yellow | 508–526 THz | 570–590 nm |
| orange | 484–508 THz | 590–620 nm |
| red | 400–484 THz | 620–750 nm |
"The visible spectrum is the portion of the electromagnetic spectrum that is visible to (can be detected by) the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm.[51] In terms of frequency, this corresponds to a band in the vicinity of 400–790 THz. A light-adapted eye generally has its maximum sensitivity at around 555 nm (540 THz), in the green region of the optical spectrum (see: luminosity function)."[52]
There are several cases of astronomers who claimed that following a cataract operation, they could see shorter wavelengths than other people, slightly into the ultraviolet.
Violets
| ||||||||
Def. “[a] bluish-purple colour"[53] is called violet.
Def. “[a] colour/color that is a dark blend of red and blue; dark magenta"[54] is called purple.
"Purple is a range of hues of color occurring between red and blue.[55] The Oxford English Dictionary describes it as a deep, rich shade between crimson and violet.[56]"[57]
Blues

"Blue is the colour of the clear sky and the deep sea.[48]"[58]
"De la couleur du ciel sans nuages, de l'azur"[59]
Cyans
| ||||||||
“Electric blue is a color close to cyan that is a representation of the color of lightning, an electric spark, and argon signs”.[60]
“The electric blue glow of electricity results from the spectral emission of the excited ionized atoms (or excited molecules) of air (mostly oxygen and nitrogen) falling back to unexcited states, which happens to produce an abundance of electric blue light. This is the reason electrical sparks in air, including lightning, appear electric blue. It is a coincidence that the color of Cherenkov radiation and light emitted by ionized air are a very similar blue despite their very different methods of production.”[60]
"Aero blue is a fluorescent cyan color."[61]
"The word [cerulean] is probably derived from the Latin word caeruleus, "dark blue, blue or blue-green", which in turn probably derives from caelulum, diminutive of caelum, "heaven, sky".[62]"[61]
"Natural gas (methane) [...] has a cyan colored flame when burned with a mixture of air."[63]
Greens
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



Green has a wavelength range of approximately 520–570 nm,[65] a frequency range of ~575–525 THz,[65] with color coordinates of (0, 255, 0) and a hexagonal triplet of #00FF00 from sRGB source of sRGB approximation to NCS S 2060-G.[66]
Def. "[t]he colour of growing foliage, as well as other plant cells containing chlorophyll; the colour between yellow and blue in the visible spectrum; one of the primary additive colour for transmitted light; the colour obtained by subtracting red and blue from white light using cyan and yellow filters"[67] is called green.
"...in nature chiefly conspicuous as the colour of growing herbage and leaves..."[68]
"Green is the color of emeralds, jade, and growing grass.[68] In the continuum of colors of visible light it is located between yellow and blue. Green is the color most commonly associated with nature and the environmental movement, Islam, spring, hope and envy.[69]"[65]
"Green is the color you see when you look at light with a wavelength of roughly 520–570 nanometers."[65]
"It is one of the three additive colors, along with red and blue, which are combined on computer screens and color televisions to make all other colors."[65]
"In the subtractive color system, used in printing, it is not a primary color, but is created out of a mixture of yellow and blue, or yellow and cyan."[65]
"On the HSV color wheel, also known as the RGB color wheel, the complement of green is magenta; that is, a purple color corresponding to an equal mixture of red and blue light. On a color wheel based on traditional color theory (RYB), the complementary color to green is considered to be red.[70]"[65]
"The perception of greenness (in opposition to redness forming one of the opponent mechanisms in human color vision) is evoked by light which triggers the medium-wavelength M cone cells in the eye more than the long-wavelength L cones. Light which triggers this greenness response more than the yellowness or blueness of the other color opponent mechanism is called green. A green light source typically has a spectral power distribution dominated by energy with a wavelength of roughly 487–570 nm. More specifically, "blue green" 487–493 nm, "bluish green" 493–498 nm, "green" 498–530 nm, "yellowish green" 530–559 nm, "yellow green" 559–570 nm.[71]"[65]
"Green earth is a natural pigment ... It s composed of clay colored by iron oxide, magnesium, aluminum silicate, or potassium. Large deposits were found in the South of France near Nice, and in Italy around Verona, on Cyprus, and in Bohemia. The clay was crushed, washed to remove impurities, then powdered. It was sometimes called Green of Verona.[72]"[65]
Yellows
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


Def. "[t]he colour of gold or butter; the colour obtained by mixing green and red light, or by subtracting blue from white light"[73] is called yellow.
Def. a "bright yellow colour, resembling the metal gold"[74]
is called
| gold. |
"Yellow, in the form of yellow ochre pigment made from clay, was one of the first colors used in prehistoric cave art. The cave of Lascaux has an image of a horse colored with yellow estimated to be 17,300 years old."[75]
Shades of yellow contains a more diverse set of yellow or yellow-like colors.
Oranges
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Def. "[t]he colour of a ripe orange (the fruit); a color midway between red and yellow"[76] is called orange.
Reds
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In wavelengths, red astronomy covers 620 - 750 nm.
"Infrared or red radiation from a common household radiator or electric heater is an example of thermal radiation, as is the heat emitted by an operating incandescent light bulb. Thermal radiation is generated when energy from the movement of charged particles within atoms is converted to electromagnetic radiation."[77]
"Infrared (IR) light is electromagnetic radiation with longer wavelengths than those of visible light, extending from the nominal red edge of the visible spectrum at 700 nanometres (nm) to 1 mm. This range of wavelengths corresponds to a frequency range of approximately 430 THz down to 300 GHz,[78] and includes most of the thermal radiation emitted by objects near room temperature. Infrared light is emitted or absorbed by molecules when they change their rotational-vibrational movements."[79]
"Far-red light is light at the extreme red end of the visible spectrum, between red and infra-red light. Usually regarded as the region between 710 and 850 nm wavelength, it is dimly visible to some [human] eyes."[80]
Auroras

"Electron temperatures are generally derived from the ratio of auroral to nebular lines in [O III] or [N II]."[82] "[B]ecause of the proximity of strong night-sky lines at λ4358 and λλ5770, 5791, the auroral lines of [O III] λ4363 and [N II] λ5755 are often contaminated."[82]
Coronal clouds
"The temperature of yellow coronal regions is ... about 2.5 [x] 106 [K]. ... although some ions Ca XV will exist at lower, as well as higher temperatures."[83]
"The AS prominences [AS in Menzel-Evans' classification [4];] move with velocities exceeding by far the velocities of other types of prominences [7], [8]. As short-living phenomena, they are condensed quickly and the temperature of the coronal gases should rise in the early stages of their condensation. Indeed, the AS prominences use to be allied with yellow line emission (λ 5694)."[83]
"The yellow line is namely due to the ion Ca XV, according to Edlen's and Waldmeier's identification. ... the line λ 5694 is emitted by 3P1 - 3P0 transition of Ca XV."[83]
"The solar corona is not in thermodynamical equilibrium. In particular, the photo-recombination is compensated with electron impact ionization, while the reverse processes viz. the photoionization and recombination by impact with two electrons are there negligible."[83]
Asteroids
"[B]roadband optical photometry of Centaurs and Kuiper Belt objects [KBOs] from the Keck 10 m, the University of Hawaii 2.2 m, and the Cerro Tololo InterAmerican (CTIO) 1.5 m telescopes [shows] a wide dispersion in the optical colors of the objects, indicating nonuniform surface properties. The color dispersion [may] be understood in the context of the expected steady reddening due to bombardment by the ubiquitous flux of cosmic rays."[84]
Kuiper belts
"These authors proposed that the whole-disk surface colors of KBOs could be the result of the competition between the effects of irradiation of surface organics by cosmic-rays and the global resurfacing due to impacts. [...] When these high-energy protons collide with an icy target, they penetrate very [deep] under the surface."[85]
Research
Hypotheses:
- The orthogonality between electric fields and magnetic fields came before 3D space.
- Orthogonality between electric fields and magnetic fields creates the illusion of three-dimensional space.
- Interference interactions create attractions and repulsions between fields which in turn produces radiance, motion, and allows time to be calibrated.
- Interactions create segmentation yielding an apparent particle/wave duality.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[86] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[87]"[88]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[89] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[90]
See also
References
- ↑ "Ultraviolet, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. 6 september 2015. Retrieved 2015-09-07.
- ↑ "Mineral exploration, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 25, 2013. Retrieved 2013-06-30.
- ↑ Stokes, G. G. (1852). "On the Change of Refrangibility of Light". Philosophical Transactions of the Royal Society of London 142: 463–562. doi:10.1098/rstl.1852.0022.
- ↑ K. Przibram (1935). "Fluorescence of Fluorite and the Bivalent Europium Ion". Nature 135 (3403): 100. doi:10.1038/135100a0.
- ↑ "Fluorite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 29, 2013. Retrieved 2013-06-30.
- ↑ D.W. Thompson, et al. (1998). "Determination of optical anisotropy in calcite from ultraviolet to mid-infrared by generalized ellipsometry". Thin Solid Films 313–4 (1-2): 341–6. doi:10.1016/S0040-6090(97)00843-2.
- ↑ "Calcite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 20, 2013. Retrieved 2013-06-30.
- ↑ "Diamond simulant, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 15, 2013. Retrieved 2013-06-30.
- 1 2 3 D. K. Yeomans (1998). "Great Comets in History". Jet Propulsion Laboratory. Retrieved 15 March 2007.
- ↑ D. A. Mendis (1988). "A Postencounter view of comets". Annual Review of Astronomy and Astrophysics 26 (1): 11–49. doi:10.1146/annurev.aa.26.090188.000303.
- ↑ Ian Ridpath (1985). "Through the comet’s tail". Revised extracts from A Comet Called Halley by Ian Ridpath, published by Cambridge University Press in 1985. Retrieved 2011-06-19.
- ↑ Brian Nunnally (May 16, 2011). "This Week in Science History: Halley’s Comet". pfizer: ThinkScience Now. Retrieved 2011-06-19.
- ↑ "Yerkes Observatory Finds Cyanogen in Spectrum of Halley's Comet". The New York Times. 8 February 1910. Retrieved 15 November 2009.
- 1 2 "Ten Notable Apocalypses That (Obviously) Didn't Happen". Smithsonian magazine. 2009. Retrieved 14 November 2009.
- ↑ "Interesting Facts About Comets". Universe Today. 2009. Retrieved 15 January 2009.
- ↑ "Halley's Comet, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. September 18, 2012. Retrieved 2012-09-19.
- ↑ Heber D. Curtis (June 1910). "Photographs of Halley's Comet made at the Lick Observatory". Publications of the Astronomical Society of the Pacific 22 (132): 117-30.
- ↑ Robert Roy Britt (2001-11-29). "Comet Borrelly Puzzle: Darkest Object in the Solar System". Space.com. Retrieved 2012-09-01.
- ↑ "Albedo, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 24, 2013. Retrieved 2013-05-01.
- ↑ Tony Hoffman. "Comet West: The Great Comet of 1976". Earthlink. Retrieved 2013-05-02.
- ↑ Abraham Loeb, Mark J. Reid, Andreas Brunthaler, and Heino Falcke (November 2005). "Constraints on the Proper Motion of the Andromeda Galaxy Based on the Survival of Its Satellite M33". The Astrophysical Journal 633 (2): 894-8. doi:10.1086/491644. http://iopscience.iop.org/0004-637X/633/2/894/fulltext. Retrieved 2011-11-14.
- ↑ The Grand Collision, from the series: The Sky At Night, airdate: November 5, 2007
- ↑ Cox, T. J.; Loeb, A. (2008). "The collision between the Milky Way and Andromeda". Monthly Notices of the Royal Astronomical Society 386 (1): 461–474. doi:10.1111/j.1365-2966.2008.13048.x.
- ↑ Cain, F. (2007). "When Our Galaxy Smashes Into Andromeda, What Happens to the Sun?". Universe Today. Retrieved 2007-05-16.
- ↑ "Andromeda Galaxy, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. September 20, 2013. Retrieved 2013-09-26.
- 1 2 S. Swordy (2001). "The energy spectra and anisotropies of cosmic rays". Space Science Reviews 99: 85–94.
- ↑ "Cosmic ray, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 17, 2012. Retrieved 2012-08-17.
- 1 2 "Neutron temperature, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 9, 2012. Retrieved 2012-08-19.
- ↑ Atoms, Radiation, and Radiation Protection, J.E. Turner, Wiley-VCH, 2007, p. 214.
- ↑ A. M. Cherepashchuk, K. F. Khaliullin, & J. A. Eaton (June 15, 1984). "Ultraviolet photometry from the Orbiting Astronomical Observatory. XXXIX - The structure of the eclipsing Wolf-Rayet binary V444 Cygni as derived from light curves between 2460 A and 3. 5 microns". The Astrophysical Journal 281 (06): 774-88. doi:10.1086/162156. http://adsabs.harvard.edu/full/1984ApJ...281..774C. Retrieved 2014-01-23.
- ↑ B. Pontecorvo (1957). "Mesonium and anti-mesonium". Zh. Eksp. Teor. Fiz. 33: 549–551. reproduced and translated in Sov. Phys. JETP 6: 429. 1957. and B. Pontecorvo (1967). "Neutrino Experiments and the Problem of Conservation of Leptonic Charge". Zh. Eksp. Teor. Fiz. 53: 1717. reproduced and translated in Sov. Phys. JETP 26: 984. 1968.
- ↑ "Neutrino oscillation, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 21, 2012. Retrieved 2012-08-23.
- ↑ Martin V. Zombeck (1990). "Calibration of MK spectral types". Handbook of Space Astronomy and Astrophysics (2nd ed.). Cambridge University Press. p. 105. ISBN 0-521-34787-4.
- ↑ David F. Gray (1992), The Inferred Color Index of the Sun, Publications of the Astronomical Society of the Pacific, vol. 104, no. 681, pp. 1035-1038 (November 1992)
- ↑ The Simbad Astronomical Database' Rigel page
- ↑ "Color index, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. November 16, 2012. Retrieved 2013-01-09.
- ↑ "Photometric system, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. November 19, 2012. Retrieved 2013-01-09.
- 1 2 James Binney; Merrifield M. Galactic Astronomy, Princeton University Press, 1998, ch. 2.3.2, pp. 53
- ↑ Webster's New World Dictionary of the American Language, Third College Edition.
- ↑ Shorter Oxford English Dictionary, 5th edition, 2002.
- ↑ "Grey, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 3, 2013. Retrieved 2013-05-02.
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- ↑ See Shorter Oxford English Dictionary, 5th Edition (2002); "of the colour of fresh milk or snow." See also Webster's New World Dictionary of American English, Third College Edition, (1988): "Having the color of pure snow or milk." See also The Random House College Dictionary of the English Language, Revised Edition,(1980)
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- 1 2 3 4 "White, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 1, 2013. Retrieved 2013-05-02.
- ↑ Petit Larousse (2005).
- 1 2 Shorter Oxford English Dictionary. 5th Edition (2002)
- 1 2 3 See also Webster's New World Dictionary of the American Language (1964)
- 1 2 Le Petit Larousse Illustré (1997)
- ↑ "Black, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 26, 2013. Retrieved 2013-05-02.
- ↑ Cecie Starr (2005). Biology: Concepts and Applications. Thomson Brooks/Cole. ISBN 053446226X. http://books.google.com/?id=RtSpGV_Pl_0C&pg=PA94.
- ↑ "Visible spectrum, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. September 15, 2012. Retrieved 2012-09-17.
- ↑ "violet, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. February 8, 2013. Retrieved 2013-03-20.
- ↑ "purple, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. February 13, 2013. Retrieved 2013-03-20.
- ↑ Mish, Frederic C., Editor in Chief Webster's Ninth New Collegiate Dictionary Springfield, Massachusetts, U.S.A.:1984--Merriam-Webster Page 957
- ↑ Shorter Oxford English Dictionary, 5th Edition, 2003.
- ↑ "Purple, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. March 19, 2013. Retrieved 2013-03-20.
- ↑ "Blue, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 28, 2013. Retrieved 2013-05-28.
- ↑ Le Petit Larousse illustré 1997. Librairie Larousse. 1996. pp. 1784. ISBN 2033011976. http://books.google.com/books?id=ZWSemgEACAAJ&hl=en&sa=X&ei=P72mUauzBoba9ASxhYHoAQ&ved=0CFQQ6AEwDw. Retrieved 2013-05-28.
- 1 2 "Electric blue (color), In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 4, 2012. Retrieved 2012-06-08.
- 1 2 "Variations of cyan, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. September 29, 2013. Retrieved 2013-10-18.
- ↑ Cerulean, Online Etymology Dictionary
- ↑ "Cyan, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 13, 2013. Retrieved 2013-10-18.
- ↑ "Malachite". WebExhibits. 2001. Retrieved 2007-12-08.
- 1 2 3 4 5 6 7 8 9 "Green, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. January 6, 2013. Retrieved 2013-01-21.
- ↑ The sRGB values are taken by converting the NCS color 2060-G using the "NCS Navigator" tool at the NCS website.
- ↑ "green, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. July 21, 2012. Retrieved 2012-07-22.
- 1 2 (Oxford English Dictionary, 2nd Edition, Clarendon Press, Oxford, 1989.) See also first definition in Webster's New World Dictionary of the American Language, The World Publishing Company, New York, 1962.
- ↑ Eva Heller, Psychologie de la couleur- effets et symboliques. pg. 87-104
- ↑ "Glossary Term: Color wheel". Sanford Corp. 2005. Archived from the original on November 2, 2007. Retrieved 2007-11-22.
- ↑ Kenneth L. Kelly (1943). "Color Designations for Lights". Journal of the Optical Society of America 33(11). 627–632.
- ↑ Anne Varichon (2000), Couleurs- pigments et teintures dans les mains des peoples. Pg. 210-211.
- ↑ "yellow". Wiktionary (San Francisco, California: Wikimedia Foundation, Inc). September 1, 2013. https://en.wiktionary.org/wiki/yellow. Retrieved 2013-09-02.
- ↑ "gold, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 28, 2013. Retrieved 2013-09-02.
- ↑ "Yellow, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. September 12, 2013. Retrieved 2013-09-16.
- ↑ "orange, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. February 20, 2014. Retrieved 2014-03-01.
- ↑ "Radiation, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 31, 2012. Retrieved 2012-06-02.
- ↑ S. C. Liew. "Electromagnetic Waves". Centre for Remote Imaging, Sensing and Processing. Retrieved 2006-10-27.
- ↑ "Infrared, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 26, 2013. Retrieved 2013-07-29.
- ↑ "Far-red, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 28, 2013. Retrieved 2013-07-29.
- ↑ S. Wolpert (July 24, 2008). "Scientists solve 30-year-old aurora borealis mystery". University of California. Retrieved 2008-10-11.
- 1 2 S. A. Hawley (September 1, 1978). "The chemical composition of galactic and extragalactic H II regions". The Astrophysical Journal 224 (9): 417-36. doi:10.1086/156389.
- 1 2 3 4 J. Kleczek (1957). "Temperature of Yellow Coronal Regions". Bulletin of the Astronomical Institutes of Czechoslovakia 8: 68-70. http://adsabs.harvard.edu/full/1957BAICz...8...68K. Retrieved 2013-09-26.
- ↑ Jane Luu and David Jewitt (November 1996). "Color Diversity among the Centaurs and Kuiper Belt Objects". The Astronomical Journal 112 (5): 2310-8. http://adsabs.harvard.edu/full/1996AJ....112.2310L. Retrieved 2013-11-05.
- ↑ R Gil-Hutton (January 2002). "Color diversity among Kuiper belt objects: The collisional resurfacing model revisited". Planetary and Space Science 50 (1): 57-62. http://www.sciencedirect.com/science/article/pii/S0032063301000733. Retrieved 2014-01-23.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
| ||||||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is an astronomy resource. |