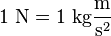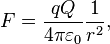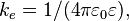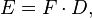Physics


Physics is a study of how the universe appears to work. It has been defined as a "science that deals with matter and energy and their interactions"[1] and as the "branch of science concerned with the study of properties and interactions of space, time, matter and energy"[2].
This resource is part of a series of lectures from the department of physics.
Time

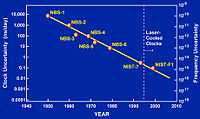

A key value on the time axis for collecting physical data is the starting time. “Incandescents reach full brightness a fraction of a second after being switched on.”[3]
"An atomic clock is a clock device that uses an electronic transition frequency in the microwave, optical, or ultraviolet region[4] of the electromagnetic spectrum of atoms as a frequency standard for its timekeeping element. Atomic clocks are the most accurate time and frequency standards known, and are used as primary standards for international time distribution services, to control the wave frequency of television broadcasts, and in global navigation satellite systems such as GPS."[5]
The FOCS 1 continuous cold cesium fountain atomic clock started operating in 2004 at an uncertainty of one second in 30 million years. The clock is in Switzerland.
Conditions
Eventually it occurred to many of the intelligent life forms on Earth that in addition to where the observations of the natural objects or entities in the sky are taken, also when and how the observations are taken is important. The results of observations change with time, temperature, and other atmospheric conditions.
Many of the early laws begin to make sense and increase understanding of the phenomena observed when coupled to experimental and theoretical studies performed in a laboratory here on Earth under controlled conditions. Astronomy benefits from physics.
Laboratory conditions are often expressed in terms of standard temperature and pressure.
"Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry (IUPAC) and the National Institute of Standards and Technology (NIST), although these are not universally accepted standards. Other organizations have established a variety of alternative definitions for their standard reference conditions."[6]
"In chemistry, IUPAC established standard temperature and pressure (informally abbreviated as STP) as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of 100 kPa (14.504 psi, 0.986 atm, 1 bar),[7] An unofficial, but commonly used standard is standard ambient temperature and pressure (SATP) as a temperature of 298.15 K (25 °C, 77 °F) and an absolute pressure of 100 kPa (14.504 psi, 0.986 atm). The STP and the SATP should not be confused with the standard state commonly used in thermodynamic evaluations of the Gibbs free energy of a reaction."[6]
"Standard conditions for gases: Temperature, 273.15 K [...] and pressure of 105 pascals. The previous standard absolute pressure of 1 atm (equivalent to 1.01325 × 105 Pa) was changed to 100 kPa in 1982. IUPAC recommends that the former pressure should be discontinued."[7]
"NIST uses a temperature of 20 °C (293.15 K, 68 °F) and an absolute pressure of 101.325 kPa (14.696 psi, 1 atm). The International Standard Metric Conditions for natural gas and similar fluids are 288.15 K (59.00 °F, 15.00 °C) and 101.325 kPa.[8]"[6]
Line of sight


Def. “[a] straight line along which an observer has a clear view”[9] is called line of sight.
In the section on 'senses' is a demonstration of the principle of 'line of sight'; i.e., "a line from an observer's eye to a distant point toward which [the observer] is looking"[1]. In the image on the left of rain beneath a dark cloud, there is a highway with a vehicle on it. The vehicle is further away from the observer than the right turn onto a side road. Is the blue sky behind the dark cloud? Is the line of trees in the background further away than the dark cloud? Many objects in this image and the others can be layered relative to the observer (some are closer by inspection than others). These layers or strata are strata along the line of sight. The principle of line of sight can be used to make deductions about the relative locations (or positions) of objects from the observer's perspective.
By observing many of the wandering lights in the night sky, an occasional occultation of the light of one astronomical object may occur by the intervention of another along a closer astronomical stratum. On April 25, 1838, an occultation of Mercury by the Moon occurred when Mercury was visible to the unaided eye after sunset.[10] An occultation of Venus by the Moon occurred "on the afternoon of October 14", 1874.[10] An earlier such occultation "occurred on May 23, 1587, and is thus recorded by [Tycho Brahe] in his Historia Celestis"[10]. "Thomas Street, in his Astronomia Carolina (A.D. 1661), mentions three occultations by Venus, being two occasions when the planet covered Regulus, and once when there was an occultation of Mars by Venus."[10] "[Thomas Street] describes [the occultation of Mars by Venus] as follows: "1590,. Oct. 2nd, 16h. 24s. Michael Mœstlin observed ♂ eclipsed by ♀.""[10]
Coordinate systems
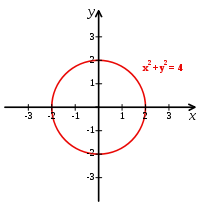
“A Cartesian coordinate system specifies each point uniquely in a plane by a pair of numerical coordinates, which are the signed distances from the point to two fixed perpendicular directed lines, measured in the same unit of length. Each reference line is called a coordinate axis or just axis of the system, and the point where they meet is its origin, usually at ordered pair (0,0). The coordinates can also be defined as the positions of the perpendicular projections of the point onto the two axes, expressed as signed distances from the origin.”[11]
Distances
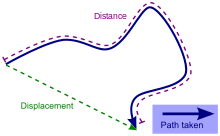
Def. an "amount of space between two points, usually geographical points, usually (but not necessarily) measured along a straight line"[12] is called a distance.
"Distance (or farness) is a numerical description of how far apart objects are. In physics or everyday discussion, distance may refer to a physical length, or an estimation based on other criteria (e.g. "two counties over"). In mathematics, a distance function or metric is a generalization of the concept of physical distance. A metric is a function that behaves according to a specific set of rules, and provides a concrete way of describing what it means for elements of some space to be "close to" or "far away from" each other."[13]
Emptiness
“In set theory, emptiness is symbolized by the empty set: a set that contains no elements.”[14]
Def. “the state of being ... [d]evoid of content; containing nothing”[15] is called empty.
“Free space, a perfect vacuum as expressed in the classical physics model ... Vacuum state, a perfect vacuum based on the quantum mechanical model ... In mathematical physics, the homogeneous equation may correspond to a physical theory formulated in empty space”[16] are disambiguations for "empty space".
“In astronomy, voids are the empty spaces between filaments (the largest-scale structures in the Universe), which contain very few, or no, galaxies. ... Voids located in high-density environments are smaller than voids situated in low-density spaces of the universe.[17]”[18]
Forces
Def. "[a] physical quantity that denotes ability to push, pull, twist or accelerate a body which is measured in a unit dimensioned in mass × distance/time² (ML/T²): SI: newton (N); CGS: dyne (dyn)" is called force.
Def. a force "associated with nuclear decay"[19] is called a weak nuclear force.
Def. "a residual force [associated with the strong bonds] responsible for the interactions between nucleons"[20] is called the strong nuclear force.
The key values to determine in both force and energy are 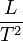 and
and 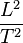 . Force (F) x distance (L) = energy (E),
. Force (F) x distance (L) = energy (E), 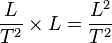 . Force and energy are related to distance and time using proportionality constants.
. Force and energy are related to distance and time using proportionality constants.
Sources
One theoretical source of a force is the presence of mass.
Newton's second law of motion is that 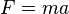 , where
, where 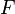 is the force applied,
is the force applied, 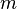 is the mass of the object receiving the force, and
is the mass of the object receiving the force, and 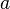 is the acceleration observed for the astronomical object. The newton is therefore:[21]
is the acceleration observed for the astronomical object. The newton is therefore:[21]
where:
- N: newton
- kg: kilogram
- m: metre
- s: second.
Every point mass attracts every single other point mass by a force pointing along the line intersecting both points. The force is proportional to the product of the two masses and inversely proportional to the square of the distance between them:[22] 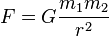 ,
,
where:
- F is the force between the masses,
- G is the gravitational constant,
- m1 is the first mass,
- m2 is the second mass, and
- r is the distance between the centers of the masses.
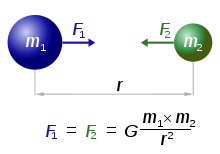 The diagram shows two masses attracting one another. Credit: .
The diagram shows two masses attracting one another. Credit: .
In the International System of Units (SI) units, F is measured in newtons (N), m1 and m2 in kilograms (kg), r in meters (m), and the constant G is approximately equal to 6.674×10−11
N m2 kg−2.[23]
Another theoretical source of a force is charge.
Coulomb's law states that the electrostatic force 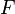 experienced by a charge,
experienced by a charge, 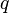 at position
at position 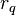 , in the vicinity of another charge,
, in the vicinity of another charge, 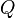 at position
at position 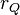 , in vacuum is equal to:
, in vacuum is equal to:
where 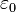 is the electric constant and
is the electric constant and  is the distance between the two charges.
is the distance between the two charges.
Coulomb's constant is
where the constant 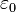 is called the permittivity of free space in SI units of C2 m−2 N−1.
is called the permittivity of free space in SI units of C2 m−2 N−1.
For reality,  is the relative (dimensionless) permittivity of the substance in which the charges may exist.
is the relative (dimensionless) permittivity of the substance in which the charges may exist.
Energy
Def. "[a] quantity that denotes the ability to do work and is measured in a unit dimensioned in mass × distance²/time² (ML²/T²) or the equivalent"[24] is called energy.
The energy 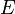 for the system in the section about forces is
for the system in the section about forces is
where 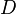 is the displacement.
is the displacement.
Fields
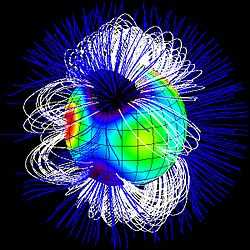
Def. "[a] region affected by a particular force" is called a field.
Def. "[a] region of space around a charged particle, or between two voltages; it exerts a force on charged objects in its vicinity"[25] is called an electric field.
Def. "a condition in the space around a magnet or electric current in which there is a detectable magnetic force and two magnetic poles are present"[26] is called a magnetic field.
Gravity
Def. the "fundamental force of attraction that exists between all particles with mass in the universe"[27] is called gravitation.
Masses
Def. "[t]he basic structural component of the universe [that] usually has mass and volume"[28] is called matter.
"In physics, mass, more specifically inertial mass, can be defined as a quantitative measure of an object's resistance to the change of its speed. In addition to this, gravitational mass can be described as a measure of magnitude of the gravitational force which is
- exerted by an object (active gravitational mass), or
- experienced by an object (passive gravitational force)
when interacting with a second object. The SI unit of mass is the kilogram (kg)."[29]
Measurements

"Measurement is the process or the result of determining the ratio of a physical quantity, such as a length, time, temperature etc., to a unit of measurement, such as the meter, second or degree Celsius."[30]
Physical units
Def. "exactly 149,597,870.691 kilometres" is called one AU.[31]
Def. "86,400 SI seconds (s)" is called 1 day (d).[31]
Def. "1.9891 x 1030 kg" is called the mass of the Sun.[31]
Def. "9,460,730,472,580.8 km" is called the light-year (ly).[31]
Fissions
"In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy, even by the energetic standards of radioactive decay. [...] The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes.[32][33] Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus."[34]
Fusions
"Nuclear fusion is a nuclear reaction in which two or more atomic nuclei come very close and then collide at a very high speed and join to form a new type of atomic nucleus. During this process, matter is not conserved because some of the matter of the fusing nuclei is converted to photons (energy). Fusion is the process that powers active or "main sequence" stars."
"The fusion of two nuclei with lower masses than iron (which, along with nickel, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements. There are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernovae."[35]
Radiations
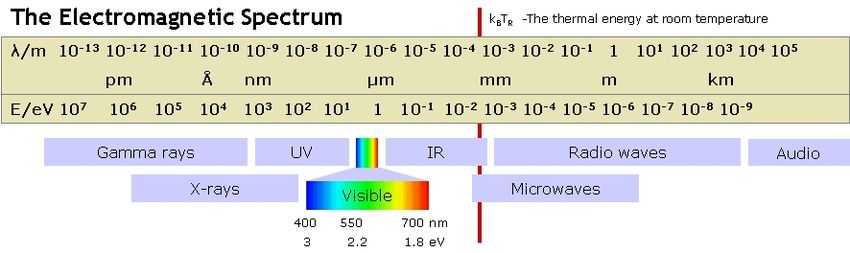
Def. an action or process of throwing or sending out a traveling ray in a line, beam, or stream of small cross section is called radiation, from radiation astronomy.
The term radiation is often used to refer to the ray itself.
Radiation comes in many forms and energies.
Notation: let various International System of Units, SI prefixes, occur before the unit of energy, the electronvolt, abbreviated as eV.
For example, PeV denotes 1015 eV.
Cosmic rays may be upwards of a ZeV (1021 eV). Ultra high energy neutrons are around an EeV (1018 eV). But, X-rays only range up to about 120 keV, while the visible (visual) range is around 2 eV.
Astronomy likely started with visual astronomy. Visual refers to that portion of the electromagnetic spectrum called the visible spectrum. Probing the sky with additional portions of this spectrum is difficult as the atmosphere absorbs over many portions.
This has produced fields of observational astronomy based on some portions of the electromagnetic spectrum:
- Gamma-ray astronomy,
- X-ray astronomy,
- Ultraviolet astronomy,
- Infrared astronomy, and
- Radio astronomy.
Recent history
The recent history period dates from around 1,000 b2k to present.
A major thrust of physics over the last few hundred years (since the time of Galileo and Newton) has been to understand the workings of the universe in terms of ever-more-fundamental principles. This has generally involved an understanding of what a "force" means, though modern formulations of forces are very different from what they were in Newton's time.
By the middle of the 20th century, physicists recognized 4 fundamental forces:
- gravity
- electromagnetism
- the "strong" nuclear force, responsible for holding nuclei together, and for nuclear power and some types of radioactivity.
- the "weak" nuclear force, responsible for some other types of radioactivity.
One unification had already taken place in the 19th century: the previously disparate electric and magnetic forces were combined into the electromagnetic force, due to the work of Oersted, Faraday, and Maxwell.
During the 20th century, all of the forces except gravity were reformulated in terms of "gauge theories" within the framework of Quantum Mechanics. The forces act through the exchange of "gauge bosons".
In the 1970's, the electromagnetic and weak forces were unified into the "electroweak force", due to the work of Weinberg, Salam, and Glashow.
In the late 20th century, a "grand unified theory" (GUT) was developed, that attempts to unify the electroweak and strong forces. This is often called the "standard model". It is still under development.
The "theory of everything" (TOE), that would unify the standard model with gravity, is still elusive. Efforts in this area include "string theory" and "supersymmetry".
Research
Hypothesis:
- Although basic physics theory has been composed on the basis of ideal constructs, many and perhaps all of these do not exist in the real universe.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[36] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[37]"[38]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[39] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[40]
See also
- Astronomy
- Astrophysics
- Radiation
- School:Physics and Astronomy
References
- 1 2 Philip B. Gove, ed (1963). Webster's Seventh New Collegiate Dictionary. Springfield, Massachusetts: G. & C. Merriam Company. pp. 1221.
- ↑ "physics, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 28, 2013. Retrieved 2013-09-30.
- ↑ "Compact fluorescent lamp, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 10. 2012. Retrieved 2012-06-16.
- ↑ Dennis McCarthy, P. Kenneth Seidelmann (2009). TIME from Earth Rotation to Atomic Physics. Weinheim: Wiley-VCH. ch. 10 & 11.
- ↑ "Atomic clock, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 12, 2012. Retrieved 2012-10-24.
- 1 2 3 "Standard conditions for temperature and pressure, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 16, 2012. Retrieved 2012-06-16.
- 1 2 Alan D. McNaught, Andrew Wilkinson (1997). Compendium of Chemical Terminology, The Gold Book (2nd ed.). Blackwell Science. ISBN 0-86542-684-8. http://books.google.com/books?id=dO5qQgAACAAJ&hl=en.
- ↑ Natural gas – Standard reference conditions (ISO 13443). Geneva, Switzerland: International Organization for Standardization. 1996. http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=20461.
- ↑ "line of sight, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. May 3, 2012. Retrieved 2012-06-16.
- 1 2 3 4 5 Samuel J. Johnson (1874). "Occultations of and by Venus". Astronomical register 12: 268-70.
- ↑ "Cartesian coordinate system, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 16, 2012. Retrieved 2012-06-16.
- ↑ "distance, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 17 April 2015. Retrieved 2015-05-11.
- ↑ "Distance, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 2, 2012. Retrieved 2012-06-16.
- ↑ "Emptiness (disambiguation), In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. January 24, 2012. Retrieved 2012-06-16.
- ↑ "empty, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. May 29, 2012. Retrieved 2012-06-16.
- ↑ "Empty space, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 13, 2012. Retrieved 2012-06-16.
- ↑ U. Lindner, J. Einasto, M. Einasto, W. Freudling, K. Fricke, E. Tago (1995). The Structure of Supervoids I: Void Hierarchy in the Northern Local Supervoid "The structure of supervoids. I. Void hierarchy in the Northern Local Supervoid". Astron. Astrophys. 301: 329. http://www.uni-sw.gwdg.de/research/preprints/1995/pr1995_14.html/ The Structure of Supervoids I: Void Hierarchy in the Northern Local Supervoid.
- ↑ "Void (astronomy), In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 2, 2012. Retrieved 2012-06-16.
- ↑ Emperorbma (22 March 2015). "weak nuclear force, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-06-25.
- ↑ "strong nuclear force, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 6 October 2013. Retrieved 2015-06-25.
- ↑ "Table 3. Coherent derived units in the SI with special names and symbols, In: The International System of Units (SI)". International Bureau of Weights and Measures. 2006.
- ↑ - Proposition 75, Theorem 35: p.956 - I.Bernard Cohen and Anne Whitman, translators: Isaac Newton, The Principia: Mathematical Principles of Natural Philosophy. Preceded by A Guide to Newton's Principia, by I.Bernard Cohen. University of California Press 1999 ISBN 0-520-08816-6 ISBN 0-520-08817-4
- ↑ "CODATA2006".
- ↑ "energy, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. July 12, 2013. Retrieved 2013-07-14.
- ↑ "electric field, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 17 January 2015. Retrieved 2015-06-25.
- ↑ "magnetic field, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 17 July 2014. Retrieved 2015-06-25.
- ↑ Petruk (23 February 2015). "gravitation, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-06-25.
- ↑ "matter, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 20, 2013. Retrieved 2013-07-04.
- ↑ "Mass, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 15, 2012. Retrieved 2012-06-16.
- ↑ "Measurement, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 14, 2012. Retrieved 2012-06-16.
- 1 2 3 4 P. K. Seidelmann (1976). "Measuring the Universe The IAU and astronomical units". The International Astronomical Union. Retrieved 2011-11-27.
- ↑ Arora, M. G.; Singh, M. (1994). Nuclear Chemistry. Anmol Publications. p. 202. ISBN 81-261-1763-X. http://books.google.com/books?id=G3JA5pYeQcgC&pg=PA202. Retrieved 2011-04-02.
- ↑ Saha, Gopal (2010). Fundamentals of Nuclear Pharmacy (Sixth ed.). Springer Science+Business Media. p. 11. ISBN 1-4419-5859-2. http://books.google.com/books?id=bEXqI4ACk-AC&pg=PA11. Retrieved 2011-04-02.
- ↑ "Nuclear fission, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. 16 June 2015. Retrieved 2015-06-25.
- ↑ "Nuclear fusion, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. 18 June 2015. Retrieved 2015-06-25.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
- African Journals Online
- Bing Advanced search
- Google Books
- Google scholar Advanced Scholar Search
- JSTOR
- Lycos search
- Office of Scientific & Technical Information
- Questia - The Online Library of Books and Journals
- SAGE journals online
- The SAO/NASA Astrophysics Data System
- Scirus for scientific information only advanced search
- SpringerLink
- Taylor & Francis Online
- Wiley Online Library Advanced Search
- Yahoo Advanced Web Search
| |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a physics resource . |