Phosphate biochemistry

Phosphate biochemistry is the study of phosphate chemical processes within, and relating to, living organisms.
Notations
Notation: let the symbols ATP, CTP, GTP, NTP, and UTP stand for adenosine triphosphate, cytidine triphosphate, guanosine triphosphate, nucleotide triphosphate, and uridine triphosphate, respectively.
Notation: let the symbols ADP, CDP, GDP, NDP, and UDP stand for adenosine diphosphate, cytidine diphosphate, guanosine diphosphate, nucleotide diphosphate, and uridine diphosphate, respectively.
Notation: let the symbols AMP, CMP, GMP, NMP, and UMP stand for adenosine monophosphate, cytidine monophosphate, guanosine monophosphate, nucleotide monophosphate, and uridine monophosphate, respectively.
Phosphates
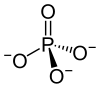
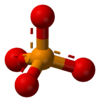
"A phosphate [is] an inorganic chemical".[1]
It "is a salt of phosphoric acid."[1]
"In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid."[1]
Biochemistry
"Biochemistry, sometimes called biological chemistry, is the study of chemical processes within, and relating to, living organisms.[2]"[3]
"Organic phosphates are important in biochemistry and biogeochemistry or ecology."[1]
"In biological systems, phosphorus is found as a free phosphate ion in solution and is called inorganic phosphate, to distinguish it from phosphates bound in various phosphate esters. Inorganic phosphate is generally denoted Pi and at physiological (neutral) pH primarily consists of a mixture of HPO42- and H2PO4- ions."[1]
Planetary sciences

"Phosphates are the naturally occurring form of the element phosphorus, found in many phosphate minerals. In mineralogy and geology, phosphate refers to a rock or ore containing phosphate ions. Inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry.[4]"[1]
Minerals





"Apatite is a group of phosphate minerals, usually referring to hydroxylapatite, fluorapatite and chlorapatite, named for high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the four most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6(F)2 and Ca10(PO4)6(Cl)2."[5]
"Apatite is one of a few minerals produced and used by biological micro-environmental systems."[5]
"Hydroxyapatite, also known as hydroxylapatite, is the major component of tooth enamel and bone mineral. A relatively rare form of apatite in which most of the OH groups are absent and containing many carbonate and acid phosphate substitutions is a large component of bone material."[5]
"Fluorapatite [a sample of which is shown at right] ... is a mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). ... Fluorapatite as a mineral is the most common phosphate mineral. It occurs widely as an accessory mineral in igneous rocks and in calcium rich metamorphic rocks. It commonly occurs as a detrital or diagenic mineral in sedimentary rocks and is an essential component of phosphorite ore deposits. It occurs as a residual mineral in lateritic soils.[6]"
At lower left is another fluorapatite example that is violet in color on quartz crystals.
"Torbernite ... is a radioactive, hydrated green copper uranyl phosphate mineral, found in granites and other uranium-bearing deposits as a secondary mineral. Torbernite is isostructural with the related uranium mineral, autunite. The chemical formula of torbenite is similar to that of autunite in which a Cu2+ cation replaces a Ca2+. The number of water hydration molecules can vary between 12 and 8, giving rise to the variety of metatorbernite when torbernite spontaneously dehydrates."[7]
"Phosphate rock can also be processed into water-soluble phosphate (P2O5) with the addition of sulfuric acid (H2SO4) to make the phosphoric acid in phosphate fertilizers. Phosphate can also be reduced in an electric furnace to make high purity phosphorus; however, this is more expensive than the acid process."[8]
Theoretical phosphate biochemistry
Def. "[a]ny salt or ester of phosphoric acid"[9] is called phosphate.
Def. any "salt or ester of pyrophosphoric acid"[10] is called a pyrophosphate.
Def. "any of a class of inorganic polymers containing linked phosphate groups"[11] is called a polyphosphate.
Entities
The catalytic phosphate in a nucleotide is usually the phosphate farthest from the nucleoside.
"The γ phosphate of ATP is the catalytic phosphate."[12]
"A phosphate which affects the catalytic activity of an enzyme is a catalytic phosphate.[13] Occasionally, an enzyme contains a structural phosphate and a catalytic phosphate.[13]"[12]
"Catalytic phosphates are acid-labile and base-stable. Structural phosphates are acid-stable, base-labile.[14]"[12]
Sources
Structural phosphate may be inside a cell, "structural to a nucleic acid such as DNA and RNA or a phospholipid. Outside the cell, phosphate may be dissolved in extracellular fluid (ECF) or form structures such as bone and teeth. Bringing phosphate in any form into the cell from a phosphate containing structure or for such a structure and when needed transporting phosphate out of the cell perhaps to a structure is a necessary activity of phosphate homeostasis for that cell."[12]
Structural phosphate is a part of each polymer whether it is a microfilament, microtubule, phospholipid, or nucleic acid. Inorganic phosphates such as Pi and PPi can be precipitated with divalent cations like Ca2+, Mg2+, or others, e.g. Mn2+, to form a variety of tissue and mineral composites: cartilage, teeth, andbone. These in turn eventually become localized to the natural environment and form phosphorites.
Bones
.jpg)
"Bones are rigid organs that constitute part of the endoskeleton of vertebrates. They support and protect the various organs of the body, produce red and white blood cells and store minerals. ... bones act as reserves of minerals important for the body, most notably calcium and phosphorus. ... bone controls phosphate metabolism by releasing fibroblast growth factor – 23 (FGF-23), which acts on kidneys to reduce phosphate reabsorption. ... The primary tissue of bone, osseous tissue, ... is mostly made up of a composite material incorporating the mineral calcium phosphate in the chemical arrangement termed calcium hydroxylapatite (this is the osseous tissue that gives bones their rigidity) and collagen, an elastic protein which improves fracture resistance."[15]
"Osteoblasts are mononucleate bone-forming cells that descend from osteoprogenitor cells. They are located on the surface of osteoid seams and make a protein mixture known as osteoid, which mineralizes to become bone. ... Osteoblasts also manufacture hormones, such as prostaglandins, to act on the bone itself. They robustly produce alkaline phosphatase, an enzyme that has a role in the mineralisation of bone, as well as many matrix proteins."[15]
"Mineralisation involves osteoblasts secreting vesicles containing alkaline phosphatase. This cleaves the phosphate groups and acts as the foci for calcium and phosphate deposition."[15]
Teeth

"A tooth (plural teeth) is a small, calcified, whitish structure found in the jaws (or mouths) of many vertebrates and used to break down food. Some animals, particularly carnivores, also use teeth for hunting or for defensive purposes. The roots of teeth are covered by gums. Teeth are not made of bone, but rather of multiple tissues of varying density and hardness. The cellular tissues that ultimately become teeth originate from the embryonic germ layer, the ectoderm".[16]
"The general structure of teeth is similar across the vertebrates, although there is considerable variation in their form and position. The teeth of mammals have deep roots, and this pattern is also found in some fish, and in crocodilians. In most teleost fish, however, the teeth are attached to the outer surface of the bone, while in lizards they are attached to the inner surface of the jaw by one side. In cartilaginous fish, such as sharks, the teeth are attached by tough ligaments to the hoops of cartilage that form the jaw.[17]"[16]
"Mammals are diphyodont, meaning that they develop two sets of teeth. In humans, the first set (the "baby," "milk," "primary" or "deciduous" set) normally starts to appear at about six months of age, although some babies are born with one or more visible teeth, known as neonatal teeth. Normal tooth eruption at about six months is known as teething and can be painful."[16]
Cartilages

Def. a "type of dense, non-vascular connective tissue, usually found at the end of joints, the rib cage, the ear, the nose, in the throat and between intervertebral disks"[18] is called cartilage.
"Ossification (or osteogenesis) is the process of laying down new bone material by cells called osteoblasts. It is synonymous with bone tissue formation. There are two processes resulting in the formation of normal, healthy bone tissue:[19] Intramembranous ossification is the direct laying down of bone into the primitive connective tissue (mesenchyme), while endochondral ossification involves cartilage as a precursor. ... Calcification is synonymous with the formation of calcium-based salts and crystals within cells and tissue. It is a process that occurs during ossification, but not vice versa."[20]
"[M]atrix vesicles (MV) are primary initiators of extracellular mineral deposition in endochondral calcification. ... direct cellular metabolism of Ca2+ and inorganic phosphate (Pi), and cellular interaction with the matrix, are involved in the formation of calcifiable MV. ... chondrocytes in growth plate (GP) cartilage ... induce the formation of calcificable MV. ... GP chondrocytes are depleted of ATP and have elevated cytosolic Pi, a condition prerequisite to formation of Ca2+-acidic phospholipid (APL)-Pi complex-primed MV. [The] interaction between the extracellular matrix and chondrocytes [may] facilitate Ca2+ loading of chondrocytes, formation of Ca2+ and Pi-primed MV and rapid induction of mineralization in GP cartilage."[21]
The spotted gar larva at right is a "living fossil" that shows cartilage beginning to be transformed into bone by endochondral calcification followed by extracellular mineralization of bone composed of calcium phosphate.
Microtubules
"During polymerization, both the α- and β-subunits of the tubulin dimer are bound to a molecule of GTP. The GTP bound to α-tubulin is stable, but the GTP bound to β-tubulin may be hydrolized to GDP shortly after assembly. The kinetics of GDP-tubulin are different from those of GTP-tubulin; GDP-tubulin is prone to depolymerization. A GDP-bound tubulin subunit at the tip of a microtubule will fall off, though a GDP-bound tubulin in the middle of a microtubule cannot spontaneously pop out. Since tubulin adds onto the end of the microtubule only in the GTP-bound state, there is generally a cap of GTP-bound tubulin at the tip of the microtubule, protecting it from disassembly. When hydrolysis catches up to the tip of the microtubule, it begins a rapid depolymerization and shrinkage. This switch from growth to shrinking is called a catastrophe. GTP-bound tubulin can begin adding to the tip of the microtubule again, providing a new cap and protecting the microtubule from shrinking. This is referred to as rescue."[22]
Microfilaments
"In vitro actin polymerization, nucleation, starts with the self-association of three G-actin monomers to form a trimer. [Adenosine triphosphate] ATP-actin then binds the plus (+) end, and the ATP is subsequently hydrolyzed with a half time of about 2 seconds[23] and the inorganic phosphate released with a half-time of about 6 minutes,[23] which reduces the binding strength between neighboring units and generally destabilizes the filament. In vivo actin polymerization is catalyzed by a new class of filament end-tracking molecular motors known as actoclampins (see next section). Recent evidence suggests that ATP hydrolysis can be prompt in such cases (i.e., the rate of monomer incorporation is matched by the rate of ATP hydrolysis)."[24]
"[Adenosine diphosphate] ADP-actin dissociates slowly from the minus end, but this process is greatly accelerated by ADP-cofilin, which severs ADP-rich regions nearest the (–)-ends. Upon release, ADP-actin undergoes exchange of its bound ADP for solution-phase ATP, thereby forming the ATP-actin monomeric units needed for further (+)-end filament elongation. This rapid turnover is important for the cell's movement. End-capping proteins such as CapZ prevent the addition or loss of monomers at the filament end where actin turnover is unfavourable like in the muscle apparatus."[24]
Rocky objects
"Phosphorite is a phosphate-rich sedimentary rock, that contains between 18% and 40% P2O5. The apatite in phosphorite is present as cryptocrystalline masses referred to as collophane."[5]
Chemistry
A phosphate reaction is a chemical reaction that leads to the transformation of one set of phosphates into another.
Each phosphate reaction that occurs in living cells is a part of phosphate metabolism. These reactions are the basis of life, allowing cells to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories: catabolism which yields energy such as the breakdown of food in cellular respiration, and anabolism which uses this energy to construct components of cells such as proteins and nucleic acids.
The KEGG[25] database contains biochemical phosphate reactions, some of which may include a nucleotide such as nucleoside monophosphate (NMP). Various possible phosphate reactions number up to 2371 as of October 20, 2009. Not all of these occur in humans. A list of biochemical phosphate reactions may contain these phosphate reactions. Those that have human genes or have products occurring in humans contribute to human life.
Catabolism
Def. "destructive metabolism, usually including the release of energy and breakdown of materials"[26] is called catabolism.
An decrease in the number of phosphates per molecule is phosphate catabolism.
For example, in phosphate catabolism, ATP is reduced to ADP with the release of an orthophosphate (Pi) and energy.
Def. any "enzyme that hydrolyzes an organic phosphate group"[27] is called a phosphohydrolase.
Def. any "of several enzymes that hydrolyze phosphate esters"[28] is called a phosphatase.
Anabolism
Def. the "constructive metabolism of the body"[29] is called anabolism.
An increase in the number of phosphates per molecule is phosphate anabolism.
For example, in phosphate anabolism, GDP is oxidized to GTP with the use of energy and the bonding of an orthophosphate (Pi) to GDP.
Def. any "enzyme that catalyzes the production of"[30] a phosphate by the addition of an inorganic phosphate to a molecule is called a phosphorylase.
Phosphoruses
"Inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry.[4]"[1]
Orthophosphoric acids
Def. "[a] colourless liquid, H3PO4"[31] is called phosphoric acid.
Def. "[o]rdinary phosphoric acid, H3PO4"[32] is called orthophosphoric acid.
"Orthophosphoric acid is a non-toxic, inorganic, rather weak triprotic acid, [that] is a very polar molecule ... soluble in water. ... Triprotic means that an orthophosphoric acid molecule can dissociate up to three times, giving up an H+ each time, which typically combines with a water molecule, H2O, as shown in these reactions:
- H3PO4(s) + H2O(l) <=> H3O+(aq) + H2PO4−(aq) Ka1= 7.25×10−3
- H2PO4−(aq)+ H2O(l) <=> H3O+(aq) + HPO42−(aq) Ka2= 6.31×10−8
- HPO42−(aq)+ H2O(l) <=> H3O+(aq) + PO43−(aq) Ka3= 3.98×10−13
The anion after the first dissociation, H2PO4−, is the dihydrogen phosphate anion. The anion after the second dissociation, HPO42−, is the hydrogen phosphate anion. The anion after the third dissociation, PO43−, is the phosphate or orthophosphate anion. For each of the dissociation reactions shown above, there is a separate acid dissociation constant, called Ka1, Ka2, and Ka3 given at 25 °C. Associated with these three dissociation constants are corresponding pKa1=2.12 , pKa2=7.21 , and pKa3=12.67 values at 25 °C."[33]
"For a given total acid concentration [A] = [H3PO4] + [H2PO4−] + [HPO42−] + [PO43−] ([A] is the total number of moles of pure H3PO4 which have been used to prepare 1 liter of solution), the composition of an aqueous solution of phosphoric acid can be calculated using the equilibrium equations associated with the three reactions described above together with the [H+][OH−] = 10−14 relation and the electrical neutrality equation. Possible concentrations of polyphosphoric molecules and ions is neglected. The system may be reduced to a fifth degree equation for [H+] which can be solved numerically, yielding:"[33]
| [A] (mol/L) | pH | [H3PO4]/[A] (%) | [H2PO4−]/[A] (%) | [HPO42−]/[A] (%) | [PO43−]/[A] (%) |
| 1 | 1.08 | 91.7 | 8.29 | 6.20×10−6 | 1.60×10−17 |
| 10−1 | 1.62 | 76.1 | 23.9 | 6.20×10−5 | 5.55×10−16 |
| 10−2 | 2.25 | 43.1 | 56.9 | 6.20×10−4 | 2.33×10−14 |
| 10−3 | 3.05 | 10.6 | 89.3 | 6.20×10−3 | 1.48×10−12 |
| 10−4 | 4.01 | 1.30 | 98.6 | 6.19×10−2 | 1.34×10−10 |
| 10−5 | 5.00 | 0.133 | 99.3 | 0.612 | 1.30×10−8 |
| 10−6 | 5.97 | 1.34×10−2 | 94.5 | 5.50 | 1.11×10−6 |
| 10−7 | 6.74 | 1.80×10−3 | 74.5 | 25.5 | 3.02×10−5 |
| 10−10 | 7.00 | 8.24×10−4 | 61.7 | 38.3 | 8.18×10−5 |
Pyrophosphoric acids
Def. the "[syrupy liquid] acid formed by the dehydration of [i.e., removing a molecule of water from] two molecules of phosphoric acid [to form one molecule of] H4P2O7"[34] is called pyrophosphoric acid.
Oligophosphoric acids
Def. a series of phosphoric acids condensed into one molecule when the number of phosphoric acids is small, per the general formula Hn+2PnO3n+1, where n is usually greater than 5, is called an oligophosphoric acid.[35]
Phosphates
"[P]hosphates are most commonly found in the form of [nucleobases such as] adenosine phosphates, ([Adenosine monophosphate] AMP, [Adenosine diphosphate] ADP and [Adenosine triphosphate] ATP) and in DNA and RNA and can be released by the hydrolysis of ATP or ADP. Similar reactions exist for the other nucleoside diphosphates and triphosphates. Phosphoanhydride bonds in ADP and ATP, or other nucleoside diphosphates and triphosphates, contain high amounts of energy which give them their vital role in all living organisms. They are generally referred to as high energy phosphate, as are the phosphagens in muscle tissue. Compounds such as substituted phosphines have uses in organic chemistry but do not seem to have any natural counterparts."[1]
Nucleotides
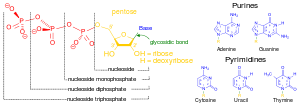
When nucleotide hydrolysis is coupled to the polymerization process, the two ends of the polymer behave differently, and the critical concentration required for assembly at one end is different from that required for the opposite end. However, NTP hydrolysis is usually associated but not directly coupled with polymerization. When there are NTP-bound subunits at the ends, the polymer is stable and continues to grow slowly. When at the polymer ends there are subunits containing NDP, the polymer is in a shrinking phase, depolymerizes rapidly and completely, and much faster at lower mer concentrations.
Phospholipids
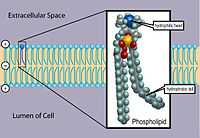
"Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from sphingosine instead of glycerol. ... The structure of the phospholipid molecule generally consists of hydrophobic tails and a hydrophilic head. Biological membranes in eukaryotes also contain another class of lipid, sterol, interspersed among the phospholipids and together they provide membrane fluidity and mechanical strength. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science.[36]"[37]
Nucleic acids
Def. "[a]ny acidic, chainlike biological macromolecule consisting of multiply repeat units of phosphoric acid, sugar and purine and pyrimidine bases"[38] occurring in cell nuclei is called a nucleic acid.
Def. a nucleic acid "in which the sugar component is threose"[39] is called threose nucleic acid, or threonucleic acid.
Materials
"Fertilizer (or fertiliser) is any organic or inorganic material of natural or synthetic origin (other than liming materials) that is added to a soil to supply one or more plant nutrients essential to the growth of plants.[40]"[8]
"John Bennet Lawes, an English entrepreneur, began to experiment on the effects of various manures on plants growing in pots in 1837, and a year or two later the experiments were extended to crops in the field. One immediate consequence was that in 1842 he patented a manure formed by treating phosphates with sulphuric acid, and thus was the first to create the artificial manure industry."[8]
"In the Nitrophosphate process or Odda Process (invented in 1927), phosphate rock with up to a 20% phosphorus (P) content is dissolved with nitric acid (HNO3) to produce a mixture of phosphoric acid (H3PO4) and calcium nitrate (Ca(NO3)2). This can be combined with a potassium fertilizer to produce a compound fertilizer with all three N:P:K: plant nutrients in easily dissolved form."[8]
"Compound fertilizers often combine N, P and K fertilizers into easily dissolved pellets. The N:P:K ratios quoted on fertilizers give the weight percent of the fertilizer in nitrogen (N), phosphate (P2O5) and potash (K2O equivalent)".[8]
"The use of phosphate fertilizers has ... increased from 9 million tonnes per year in 1960 to 40 million tonnes per year in 2000. A maize crop yielding 6–9 tonnes of grain per hectare requires 31–50 kg of phosphate fertilizer to be applied, soybean requires 20–25 kg per hectare.[41]"[8]
"Common agricultural grade phosphate fertilizers usually contain impurities such as fluorides, cadmium and uranium, although concentrations of the latter two heavy metals are dependent on the source of the phosphate and the production process."[8]
"The most widely used inorganic fertilizer is super-phosphate and its double and triple strengthed derivatives double super and triple super. Super phosphate was first developed by Lawes at the Rothamstead Agricultural Research Institute in England in the early 19th Century.[42] Lawes added sulfuric acid to conventional rock phosphate containing the mineral apatite, a calcium fluoro-phosphate. The resulting water soluble phosphorus was able to significantly improve yields on a variety of crops at the Rothamstead Centre and the Superphosphate industry was born."[8]
"[T]he citric acid solubility of phosphate rocks has emerged as a measure of plant availability and enabled so-called 'reactive' phosphate rocks to be used as fertilizer minerals. These should not be confused with high fluorine apatite rocks in which the fluoride content performs a similar function to its role in hardening teeth enamel, i.e. immobilizing phosphorus. This explains the oceanic origins of many of these high fluorine rocks (Christmas Island, Ocean Island) since the fluorine absorbed from the sea has prevented what were originally massive deposits of bird guano - from being leached from the coral based limestone rocks on which they were originally deposited."[8]
"Many inorganic fertilizers, particularly those based on superphosphate, may not replace trace mineral elements in the soil which become gradually depleted by crops. This depletion has been linked to studies which have shown a marked fall (up to 75%) in the quantities of such minerals present in fruit and vegetables.[43] Explanations for this include the early encouragement of so-called "luxury consumption" of trace elements as a result of their acidulation and subsequent dissolution in soil water, by free sulphuric acid sourced from superphosphate. This mechanism has also been identified as a possible causal agent for take-up of the heavy metal cadmium from superphosphate based fertilizers."[8]
"Organic fertilizers include naturally occurring organic materials, (e.g. chicken litter, manure, worm castings, compost, seaweed, guano, bone meal) or naturally occurring mineral deposits (e.g. [sodium nitrate] saltpeter). Poultry litter and cattle manure often create environmental and disposal problems, making their use as fertilizer beneficial. Bones can be processed into phosphate-rich bone meal; however, most are simply buried in landfills."[8]
"Even if all bones, human, animal and plant wastes were recovered to the extent practical and used for fertilizer, mineral fertilizers and synthetic nitrogen [may] still be required to make for losses to leaching, to the atmosphere, runoff and the losses impractical to recover."[8]
Rock phosphate is inorganic (not of biologic origins) and "energetically intensive to harvest and ... approved for usage in organic agriculture in minimal amounts.[44][45][46]"[8]
"The concentration of up to 100 mg/kg of cadmium in phosphate minerals (for example, minerals from Nauru[47] and the Christmas islands[48]) increases the contamination of soil with cadmium, for example in New Zealand.[49]"[8]
"Uranium is another example of a contaminant often found in phosphate fertilizers (at levels from 7 to 100 pCi/g).[50] Eventually these heavy metals can build up to unacceptable levels and build up in vegetable produce.[49] Average annual intake of uranium by adults is estimated to be about 0.5 mg (500 μg) from ingestion of food and water and 0.6 μg from breathing air.[51]"[8]
"Also, highly radioactive Polonium-210 contained in phosphate fertilizers is absorbed by the roots of plants and stored in its tissues; tobacco derived from plants fertilized by rock phosphates contains Polonium-210 which emits alpha radiation estimated to cause about 11,700 lung cancer deaths each year worldwide.[52][53][54][55][56][57]"[8]
Ecology
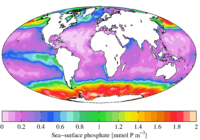
"In ecological terms, because of its important role in biological systems, phosphate is a highly sought after resource. Once used, it is often a limiting nutrient in environments, and its availability may govern the rate of growth of organisms."[1]
Research
Hypothesis:
- Polyphosphate begins the process of forming nucleic acids.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[58] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[59]"[60]
A control group for phosphate biochemistry may be a biochemistry group that does not include phosphate with the test group being one that hopefully shows a statistically systematic change from the biochemistry group.
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[61] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[62]
See also
References
- 1 2 3 4 5 6 7 8 9 "Phosphate, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 10, 2013. Retrieved 2013-04-21.
- ↑ http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_ARTICLEMAIN&node_id=1188&content_id=CTP_003379&use_sec=true&sec_url_var=region1&__uuid=aa3f2aa3-8047-4fa2-88b8-32ffcad3a93e
- ↑ "Biochemistry, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 15, 2013. Retrieved 2013-04-21.
- 1 2 "Phosphate Primer".
- 1 2 3 4 "Apatite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 9, 2013. Retrieved 2013-08-22.
- ↑ http://rruff.geo.arizona.edu/doclib/hom/fluorapatite.pdf Mineral Handbook
- ↑ "Torbernite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 27, 2013. Retrieved 2013-05-08.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "Fertilizer, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 17, 2013. Retrieved 2013-04-21.
- ↑ "phosphate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 19, 2013. Retrieved 2013-08-23.
- ↑ "pyrophosphate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 18, 2013. Retrieved 2013-08-23.
- ↑ "polyphosphate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 18, 2013. Retrieved 2013-08-23.
- 1 2 3 4 Henry A. Hoff (14 June 2009). "Phosphate reserves, In: Wiki Doc". Boston, Massachusetts: WikiDoc Foundation. Retrieved 2014-12-09.
- 1 2 Linn TC, Srere PA (March 1979). "Identification of ATP citrate lyase as a phosphoprotein". J Biol Chem. 254 (5): 1691-8. PMID 762167.
- ↑ Janski AM, Srere PA, Cornell NW, Veech RL (October 1979). "Phosphorylation of ATP Citrate Lyase in Response to Glucagon". J Biol Chem. 254 (19): 9365-8. PMID 489538.
- 1 2 3 "Bone, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 20, 2013. Retrieved 2013-08-22.
- 1 2 3 "Tooth, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 23, 2013. Retrieved 2013-08-23.
- ↑ Alfred Sherwood Romer, Thomas S. Parsons (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 300–10. ISBN 0-03-910284-X.
- ↑ "cartilage, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. July 13, 2013. Retrieved 2013-08-23.
- ↑ Caetano-Lopes J, Canhão H, Fonseca JE (2007). "Osteoblasts and bone formation". Acta reumatológica portuguesa 32 (2): 103–10. PMID 17572649.
- ↑ "Ossification, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 7, 2013. Retrieved 2013-08-23.
- ↑ Roy E. Wuthier (1993). "Involvement of Cellular Metabolism of Calcium and Phosphate in Calcification of Avian Growth Plate Cartilage". The Journal of Nutrition 123 (2 Supplement): 301-9. PMID 8429379. http://europepmc.org/abstract/MED/8429379. Retrieved 2013-08-23.
- ↑ Alexandra Almonacid E. (August 9, 2012). "Microtubule, In: Wiki Doc". Boston, Massachusetts: WikiDoc Foundation. Retrieved 2013-08-23.
- 1 2 Pollard T. D., Earnshaw W. D. (2004). Cell Biology (First Edition ed.). SAUNDERS. ISBN 1-4160-2388-7.
- 1 2 24.243.155.10 (September 4, 2012). "Microfilament, In: Wiki Doc". Boston, Massachusetts: WikiDoc Foundation. Retrieved 2013-08-23.
- ↑ Kanehisa Laboratory (September 1991). "GenomeNet KEGG database". Kyoto, Japan: Kyoto University Bioinformatics Center. Retrieved 2013-08-22.
- ↑ "catabolism, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 18, 2013. Retrieved 2013-08-23.
- ↑ "phosphohydrolase, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 23 August 2013. Retrieved 2013-08-23.
- ↑ "phosphatase, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 17, 2013. Retrieved 2013-08-23.
- ↑ "anabolism, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. May 16, 2013. Retrieved 2013-08-23.
- ↑ "phosphorylase, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 17, 2013. Retrieved 2013-08-23.
- ↑ "phosphoric acid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 17, 2013. Retrieved 2013-08-23.
- ↑ "orthophosphoric acid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 16 June 2013. Retrieved 2015-03-25.
- 1 2 "Phosphoric acid, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 19, 2013. Retrieved 2013-08-22.
- ↑ "pyrophosphoric acid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 18, 2013. Retrieved 2013-08-23.
- ↑ E. Wiberg, N. Wiberg, and A. F. Holleman (2001). Inorganic Chemistry. Academic Press. pp. 1884.
- ↑ Mashaghi S., Jadidi T., Koenderink G., Mashaghi A. (2013). "Lipid Nanotechnology". Int. J. Mol. Sci. 2013 (14): 4242–4282. doi:10.3390/ijms14024242. http://www.mdpi.com/1422-0067/14/2/4242.
- ↑ "Phospholipid, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 1, 2013. Retrieved 2013-08-23.
- ↑ "nucleic acid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. January 12, 2013. Retrieved 2013-04-19.
- ↑ "threose nucleic acid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 14, 2012. Retrieved 2013-04-19.
- ↑ "Glossary of Soil Science Terms". Soil Science Society of America. Retrieved May 10, 2011.
- ↑ Carroll P. Vance, Uhde-Stone & Allan (2003). "Phosphorus acquisition and use: critical adaptations by plants for securing a non renewable resource". New Phythologist (Blackwell Publishing) 157 (3): 423–47. doi:10.1046/j.1469-8137.2003.00695.x.
- ↑ "Lawes, Sir John Bennet (1814-1900) English Agriculturist (Scientist)". What-when-how.com. Retrieved 2013-01-03.
- ↑ Felicity Lawrence (2004). "214". In Kate Barker. Not on the Label. Penguin. p. 213. ISBN 0-14-101566-7.
- ↑ "Organic Agriculture". Google.com. Retrieved 2012-06-17.
- ↑ "Can I Use This Input on My Organic Farm?". eXtension. Retrieved 2010-08-25.
- ↑ Alternative Farming Systems Information Center. "Organic Production and Organic Food: Information Access Tools". Nal.usda.gov. Retrieved 2010-08-25.
- ↑ Syers JK, Mackay AD, Brown MW, Currie CD (1986). "Chemical and physical characteristics of phosphate rock materials of varying reactivity". J Sci Food Agric 37 (11): 1057–64. doi:10.1002/jsfa.2740371102.
- ↑ Trueman NA (1965). "The phosphate, volcanic and carbonate rocks of Christmas Island (Indian Ocean)". Journal of the Geological Society of Australia 12: 261–86.
- 1 2 Taylor MD (1997). "Accumulation of Cadmium derived from fertilizers in New Zealand soils". Science of Total Environment 208: 123–26. doi:10.1016/S0048-9697(97)00273-8.
- ↑ "Radiation Protection:Fertilizer and Fertilizer Production Wastes". US EPA. March 11, 2009. Retrieved February 2, 2010.
- ↑ "Depleted uranium: Intake of depleted uranium". World Health Organization (WHO). January 2003. Retrieved February 2, 2010.
- ↑ Hussein EM (1994). "Radioactivity of phosphate ore, superphosphate, and phosphogypsum in Abu-zaabal phosphate". Health Physics 67 (3): 280–2. doi:10.1097/00004032-199409000-00010. PMID 8056596.
- ↑ Barisic D, Lulic S, Miletic P (1992). "Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters". Water Research 26 (5): 607–11. doi:10.1016/0043-1354(92)90234-U.
- ↑ Scholten LC, Timmermans CWM (1992). "Natural radioactivity in phosphate fertilizers". Nutrient cycling in agroecosystems 43: 103–7. doi:10.1007/BF00747688.
- ↑ American Public Health Association, Framing Health Matters, Waking a Sleeping Giant: The Tobacco Industry’s Response to the Polonium-210 Issue: Monique E. Muggli, MPH, Jon O. Ebbert, MD, Channing Robertson, PhD and Richard D. Hurt, MD
- ↑ Journal of the Royal Society of Medicine, The big idea: polonium, radon and cigarettes, Tidd J R Soc Med.2008; 101: 156–157
- ↑ The Age Melbourne Australia, Big Tobacco covered up radiation danger, William Birnbauer
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
- African Journals Online
- Bing Advanced search
- Google Books
- Google scholar Advanced Scholar Search
- JSTOR
- Lycos search
- NCBI All Databases Search
- NCBI Site Search
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- Questia - The Online Library of Books and Journals
- SAGE journals online
- Scirus for scientific information only advanced search
- SpringerLink
- Taylor & Francis Online
- WikiDoc The Living Textbook of Medicine
- Wiley Online Library Advanced Search
- Yahoo Advanced Web Search
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a biochemistry resource. |
| |
Subject classification: this is a chemistry resource . |
| |
Subject classification: this is a Geology resource. |
| |
Subject classification: this is a materials science resource. |
| |
Subject classification: this is a medicine resource. |
| |
Subject classification: this is a technology resource . |