Minerals/Metals/Body-centered cubics
< Minerals < Metals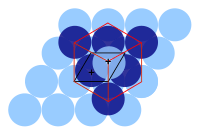
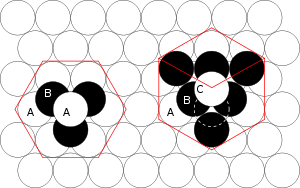
Metals in general fall into two groups of close-packing structures: face-centered cubic (fcc) and hexagonal close-packed (hcp). These two are the most efficient way to pack a bunch of hard marbles into the smallest space.
An fcc structure differs from a hcp in the number of distinct close-packed layers: hcp has two, symbolized with A below B, and fcc which has three with A below B, but C above B before A again.
Body-centered cubics
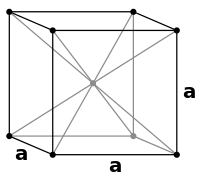
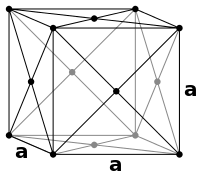
The body-centered cubic (bcc) metals have a structure for their unit cells shown in the diagram on the left. This is not a close-packed structure. As such it is expected to occur in close-packed structures at higher temperatures.
Many pure element metals occur in a bcc structure:
- α-Cr,
- α-Fe and δ-Fe,
- β-Hf,
- α-Li,
- α-Mn and δ-Mn,
- α-Mo,
- α-Nb,
- α-Ta,
- β-Ti,
- α-V,
- α-W, and
- β-Zr.
The minerals that are or contain these metals that crystallize in a bcc lattice: titanium (Ti) through chromium (Cr), zirconium (Zr) through molybdenum (Mo), and hafnium (Hf) through tungsten (W) are studied here.
Titaniums
"Microbeam analysis of eclogites from the ultrahigh-pressure metamorphic belt in Dabieshan, China has revealed native titanium inclusions in garnets of coesite eclogite. The inclusions are about 10 μm in size, have a submetallic luster from the thin oxidation film on the surface, and are brown under reflected light."[1]
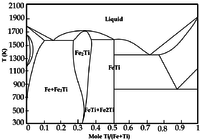
Titanium "undergoes a phase transformation (hcp to bcc) at 882 °C [5]."[2]
As the phase diagram on the left indicates, there is a miscibility gap between bcc iron (α-Fe) and hcp (α-Ti) up to about 800°C.
Vanadiums
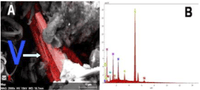
"[N]ative vanadium [occurs] in natural fumarolic incrustations and in the mineral assemblage precipitated in silica tubes inserted into high-temperature (750-830°C) fumaroles of Colima volcano – the most active volcano of Mexico, and one of the most active in the Americas. [...] The new mineral and its name (“vanadium”) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (Williams et al., 2013; IMA # 2012- 021a). The holotype material has been deposited in the Geological Museum of National Mexican University (New mineral collection of Mexican Mineralogical Society with cataloged under FIM 12/01)."[3]
In the image on the right, the backscattered electron micrograph on the left side, has the native vanadium crystals colorized in red. The energy dispersive X-ray spectroscopy (EDS) spectrum on the right shows the vanadium peaks plus small amounts of Fe and S.[3]
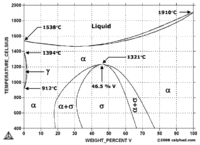
As the phase diagram on the left indicates vanadium is bcc down to lower temperatures from its melting point.
Chromiums

Native chromium such as the nugget in the image on the right is very rare. It is also a hard mineral, probably because of an oxide coating giving it a slight bluish cast.
"An unusual mineral association (diamond, SiC, graphite, native chromium, Ni-Fe alloy, Cr2+-bearing chromite), indicating a high-pressure, reducing environment, occurs in both the peridotites and chromitites."[4]
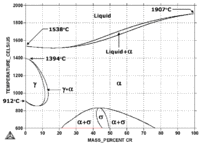
As the phase diagram for the Fe-Cr system on the left shows, chromium is bcc from 600°C on up to melting. Chromium is also bcc at room temperature and pressure.
Zirconiums
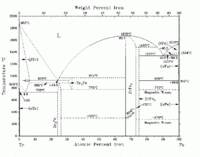
As the Fe-Zr phase diagram on the left demonstrates, zirconium has a hcp structure (α-Zr) at lower temperatures, including room temperature, and a bcc structure (β-Zr) at higher temperatures up to melting.
Niobiums
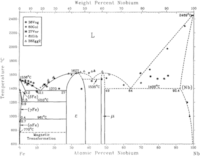
As can be seen in the iron-niobium phase diagram on the left, niobium is single phase (α-Nb) up to its melting temperature. This is a bcc structure.
It appears to be the case that native niobium does not occur in the surface rocks on Earth.
Molybdenums

The electron micrograph on the right shows a couple of pieces of native molybdenum found in lunar regolith at the Luna 24 landing site after transport back to Earth and analysis.
The phase diagram for the iron-molybdenum system demonstrates that molybdenum is bcc (α-Mo) for its intermediate and higher temperatures. It's also bcc at room temperature.
Hafniums
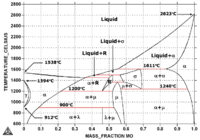
Note in the iron-hafnium phase diagram on the left that hafnium occurs in two phases: hcp (α-Hf) at lower temperatures and bcc (β-Hf) at higher temperatures up to melting.
Tantalums

On the right is a scanning electron micrograph of a piece of native tantalum from Kvanefjeld Mountain, Kuannersuit Plateau, Ilímaussaq complex, Narsaq, Kujalleq, Greenland.
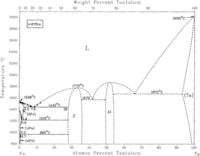
The iron-tantalum phase diagram on the left shows the bcc (α-Ta) phase from lower temperatures through and up to melting.
Tungstens

In the scanning electron micrograph on the right is a bright grain, or crystalline mass, of native tungsten. The sample is a fragment of lunar silicate glass from the Luna 24 landing site, Mare Crisium, The Moon. The fragment is bright in backscattered electrons.
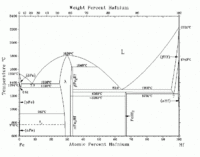
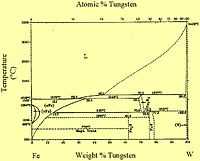
The iron-tungsten phase diagram on the left shows that the bcc phase of tungsten (α-W) occurs from lower temperatures on up to the melting temperature.
Research
Hypothesis:
- Taking on a bcc structure to defer melting to a higher temperature came before a fcc or hcp structure.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[5] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[6]"[7]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[8] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[9]
See also
References
- ↑ Jing Chen, Jiliang Li, and Jun Wu (30 April 2000). "Native titanium inclusions in the coesite eclogites from Dabieshan, China". Earth and Planetary Science Letters 177 (3-4): 237-40. doi:10.1016/S0012-821X(00)00057-1. http://www.sciencedirect.com/science/article/pii/S0012821X00000571. Retrieved 2015-08-19.
- ↑ B.B. Panigrahi, M.M. Godkhindi , K. Das, P.G. Mukunda, and P. Ramakrishnan (15 April 2005). "Sintering kinetics of micrometric titanium powder". Materials Science and Engineering: A 396 (1-2): 255-62. doi:10.1016/j.msea.2005.01.016. http://www.sciencedirect.com/science/article/pii/S0921509305000778. Retrieved 2015-08-19.
- 1 2 MikhailI Ostrooumov and Yuri Taran (20 May 2015). "Discovery of Native Vanadium, a New Mineral from the Colima Volcano, State of Colima (Mexico)". Revista de la Sociedad Española de Mineralogía: 109-10. http://www.uhu.es/fexp/sem2015/arc/macla/macla_20_109-110.pdf. Retrieved 2015-08-19.
- ↑ Wen-Ji Bai, Mei-Fu Zhou, and Paul T. Robinson (August 1993). "Possibly diamond-bearing mantle peridotites and podiform chromitites in the Luobusa and Donqiao ophiolites, Tibet". Canadian Journal of Earth Sciences 30 (8): 1650-9. doi:10.1139/e93-143. http://www.nrcresearchpress.com/doi/abs/10.1139/e93-143. Retrieved 2015-08-19.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a Geology resource. |