Minerals

Minerals are usually solid, inorganic substances of natural occurrence.
Materials
Def. matter "which may be shaped or manipulated, particularly in making something"[1] is called a material.
On the right are displayed tiles of naturally occurring materials. Wood is a mineraloid.
Theoretical minerals
Def. any "naturally occurring inorganic material that has a (more or less) definite chemical composition and characteristic physical properties"[2] is called a mineral.
Silicate minerals

Silicate minerals are those with more atomic percent silicon oxide than other constituent elements.
For example, kaolinite is Al2Si2O5(OH)4. If aluminum (Al) at two atoms per molecular unit were the most numerous element, kaolinite would be an aluminide.
As the most numerous element is oxygen at 9 for 52.9 at %, kaolin is an oxide. The crystal structure consists of a sheet of interconnected silica tetrahedra. So, kaolin is a phyllosilicate.
Def. the "oxyanion of silicon SiO32- or any salt or mineral containing this ion"[3] is called a metasilicate.
Def. "any simple silicate mineral in which the SiO4 tetrahedra are isolated and have metal ions as neighbours"[4] is called a neosilicate.
Def. a "type of silicate crystal structure characterized by the linking of SiO4 tetrahedra through other cations rather than the sharing of oxygens among SiO4 tetrahedra"[5] is called a nesosilicate.
Def.
- "any salt or ester of orthosilicic acid, (M+)4SiO44− or Si(OR)4"[6] or
- "any silicate mineral, such as garnet or olivine, in which the SiO4 tetrahedra do not share oxygen atoms with each other"[6]
is called an orthosilicate.
Def. any group of silicates that have structurally isolated double tetrahedra (the dimeric anion Si2O76-)[7] is called a sorosilicate.
Def. any group of silicates that have a ring of linked tetrahedra is called a cyclosilicate.
Def. "any silicate having interlocking chains of silicate tetrahedra"[8] is called an inosilicate.
Def. any "silicate mineral having a crystal structure of parallel sheets of silicate tetrahedra"[9] is called a phyllosilicate.
Def. type "of silicate crystal structure characterized by the sharing of all SiO4 tetrahedral oxygens resulting in three-dimensional framework structures"[5] is called a tektosilicate.
Def. any "of various silicate minerals ... with a three-dimensional framework of silicate tetrahedra"[10] is called a tectosilicate.
Alpha quartzes
Def. "a continuous framework [tectosilicate] of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall [chemical] formula [of] SiO2 ... [of] trigonal trapezohedral class 3 2"[11], usually with some substitutional or interstitial impurities, is called α-quartz.
When the concentration of interstitial or substitutional impurities becomes sufficient to change the space group of a mineral such as α-quartz, the result is another mineral. When the physical conditions are sufficient to change the solid space group of α-quartz without changing the chemical composition or formula, another mineral results.
"Shocked quartz is associated with two high pressure polymorphs of silicon dioxide: coesite and stishovite. These polymorphs have a crystal structure different from standard quartz. Again, this structure can only be formed by intense pressure, but moderate temperatures. High temperatures would anneal the quartz back to its standard form."[12]
Beta quartzes
Beta quartz (β-Quartz) is stable "between 573° and 870°C".[5]
Seifertites
Def. a polymorph of α-quartz formed at an estimated minimum pressure of 35 GPa up to pressures above 40 GPa with a orthorhombic space group Pmmm no. 47 is called seifertite.[13]
Tridymites

Def. a polymorph of α-quartz formed at temperatures from 22-460°C with at least seven space groups for its forms with tabular crystals is called tridymite.[14]
Coesites
Alpha-quartz (space group P3121, no. 152, or P3221, no. 154) under a high pressure of 2-3 gigapascals and a moderately high temperature of 700°C changes space group to monoclinic C2/c, no. 15, and becomes the mineral coesite.
Coesite is "found in extreme conditions such as the impact craters of meteorites"[15].
Stishovites
Def. a polymorph of α-quartz formed by pressures > 100 kbar or 10 GPa and temperatures > 1200 °C is called stishovite.[16]
Stishovite may be formed by an instantaneous over pressure such as by an impact or nuclear explosion type event.[12]
"[M]inute amounts of stishovite has been found within diamonds[17]"[16].
Cristobalites

Def. a high-temperature (above 1470°C) polymorph of α-quartz with cubic, Fd3m, space group no. 227, and a tetragonal form (P41212, space group no. 92) is called cristobalite.[18]
Sodalites

"Sodalite is a rich royal blue mineral ... massive sodalite samples are opaque, crystals are usually transparent to translucent. ... Occurring typically in massive form, sodalite is found as vein fillings in plutonic igneous rocks such as nepheline syenites."[19]
Lazurites

"Lazurite is a tectosilicate mineral with sulfate, sulfur and chloride with formula: (Na,Ca)8[(S,Cl,SO4,OH)2|(Al6Si6O24)]. It is a feldspathoid and a member of the sodalite group. ... The colour is due to the presence of S3- anions. ... Lazurite is a product of contact metamorphism of limestone"[20]
Glaucophanes

Often a mineral appears blue due to the presence of copper or sulfur. Glaucophane is a blue silicate that owes its color to its characteristic formation.
"Glaucophane is a mineral belonging to the amphibole group, chemical formula Na2Mg3Al2Si8O22(OH)2 ... The blue color is very diagnostic for this species. It, along with the closely related mineral riebeckite are the only common amphibole minerals that are typically blue. ... Glaucophane forms in metamorphic rocks that are either particularly rich in sodium or that have experienced low temperature-high pressure metamorphism such as would occur along a subduction zone. This material has undergone intense pressure and moderate heat as it was subducted downward toward the mantle. It is glaucophane's color that gives the blueschist facies its name. Glaucophane is also found in eclogites that have undergone retrograde metamorphism.[21]"[22]
Hauynes

"Hauyne, haüyne or hauynite [occurs] in Vesuvian lavas in Monte Somma, Italy,[23] ... It is a tectosilicate mineral with sulfate, with endmember formula Na3Ca(Si3Al3)O12(SO4).[24] ... It is a feldspathoid and a member of the sodalite group.[25][26] ... Haüyne occurs in phonolites and related leucite- or nepheline-rich, silica-poor, igneous rocks; less commonly in nepheline-free extrusives[27][25][26][28] and metamorphic rocks (marble).[25]"[29]
Olivines


Def. "[a]ny of a group of olive green magnesium-iron" orthosilicates "that crystallize in the orthorhombic system"[30] is called an olivine.
At right is a visual close up of green sand which is actually olivine crystals that have been eroded from lava rocks. Some olivine crystals are still inside the lava rock.
"Forsterite (Mg2SiO4) is the magnesium rich end-member of the olivine solid solution series."[31]
"Forsterite is associated with igneous and metamorphic rocks and has also been found in meteorites. In 2005 it was also found in cometary dust returned by the Stardust probe.[32] In 2011 it was observed as tiny crystals in the dusty clouds of gas around a forming star.[33]"[31]
"Two polymorphs of forsterite are known: wadsleyite (also orthorhombic) and ringwoodite (isometric). Both are mainly known from meteorites."[31]
Chlorites
"The name [greenstone] comes from the green hue imparted by the colour of the metamorphic minerals within the mafic rocks. Chlorite, actinolite and other green amphiboles are the typical green minerals."[34]
Pyroxenes

Def. a group of monoclinic or orthorhombic, single chain inosilicates with the general formula of X Y(Si,Al)2O6, where
- X is calcium, sodium, ferrous iron (Fe2+), magnesium, zinc, manganese and lithium;
- Y is chromium, aluminum, ferric iron (Fe3+), magnesium, manganese, scandium, titanium, vanadium, and ferrous iron (Fe2+)
is called a pyroxene.
"The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. They share a common structure consisting of single chains of silica tetrahedra and they crystallize in the monoclinic and orthorhombic systems. Pyroxenes have the general formula XY(Si,Al)2O6 (where X represents calcium, sodium, iron+2 and magnesium and more rarely zinc, manganese and lithium and Y represents ions of smaller size, such as chromium, aluminium, iron+3, magnesium, manganese, scandium, titanium, vanadium and even iron+2). Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes."[35]
At right is an image of a very rare, sharp, complete-all-around pyroxene is from Ducktown District, Polk County, Tennessee, USA, circa mid to late 1800s.
Amphiboles
Def. a group of monoclinic or orthorhombic double chain inosilicates with the general formula of
- X2Y5Z8O22(OH)2 where
- X is magnesium, ferrous iron (Fe2+), calcium, lithium, sodium, and ferric iron (Fe3+)
- Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc.
- Z is chiefly Si or Al
is called an amphibole.
Micas

Def. a group of monoclinic phyllosilicates with the general formula[36]
- X2Y4–6Z8O20(OH,F)4
- in which X is K, Na, or Ca or less commonly Ba, Rb, or Cs;
- Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc.;
- Z is chiefly Si or Al, but also may include Fe3+ or Ti;
- dioctahedral (Y = 4) and trioctahedral (Y = 6)
is called a mica.
Feldspars
Def. "[a]ny of a large group of ... aluminum [tectosilicates] of the alkali metals sodium, potassium, calcium and barium"[37] is called feldspar.
Plagioclases
Def. "[a]ny of a group of aluminum silicate feldspathic minerals ranging in their ratio of calcium to sodium"[38] is called plagioclase.
Mineraloids

Def. "[a] substance that resembles a mineral but does not exhibit crystallinity"[39] is called a mineraloid.
Def. a "chemical element existing in an uncombined state in nature"[5] is called a native element.
Def. a naturally occurring, silvery-colored, metallic liquid, composed primarily of the chemical element mercury, is called mercury, or native mercury.
Def. "[a] hard, generally yellow to brown translucent fossil resin"[40] is called an amber.
Def. "[a] flammable liquid ranging in color from clear to very dark brown and black, consisting mainly of hydrocarbons"[41] is called petroleum.
Def. a naturally occurring black glass is called an obsidian. An example is in the image on the right.
Def. "[a] small, round, dark glassy object, composed of silicates"[42] is called a tektite.
Def. a naturally occurring, hydrous, "amorphous form of silica, ... [where] 3% to 21% of the total weight is water"[43] is called an opal.
Moldavites
"Moldavite ... is an olive-green or dull greenish vitreous substance possibly formed by a meteorite impact. It is one kind of tektite."[44]
"Because of their difficult fusibility, extremely low water content, and its chemical composition, the current overwhelming consensus among Earth scientists is that moldavites were formed 15 million years ago during the impact of a giant meteorite in present-day Nördlinger Ries. Splatters of material that was melted by the impact cooled while they were actually airborne and most fell in central Bohemia—traversed by [the] Vltava river ... Currently, moldavites have been found in [an] area that includes southern Bohemia, western Moravia, the Cheb Basin (northwest Bohemia), Lusatia (Germany), and Waldviertel (Austria).[45] Isotope analysis of samples of moldavites have shown a beryllium-10 isotope composition similar to the composition of Australasian tektites (Australites)and Ivory Coast tektites (Ivorites). Their similarity in beryllium-10 isotope composition indicates that moldavites, Australites, and Ivorites consist of near surface and loosely consolidated terrestrial sediments melted by hypervelocity impacts.[46]"[44]
Rocks

Rocks are a bound aggregate of minerals with usually a large geographic extent.
Occasionally, a rock is composed of only one mineral. But a crystal of the mineral fluorite in your hand is a stone rather than a rock.
Def.
- a "naturally occurring aggregate of solid mineral matter that constitutes a significant part of the earth's crust"[47] or
- any "natural material with a distinctive composition of minerals"[47]
is called a rock.
Def. full "of, or abounding in, rocks; consisting of rocks"[48] is called rocky.
Mineralogy
Def.
- the scientific study of minerals or
- "a science dealing with minerals, their crystallography, physical and chemical properties, classification, and the ways of distinguishing them"[49]
is called mineralogy.
Def. the "experimental science of determining the arrangement of atoms in solids"[50] is called crystallography.
Aluminides

The aluminides are those naturally occurring minerals with a high atomic % aluminum.
In the image on the right of a flake of native aluminum, the scale bar = 1 mm.
"Aluminium is the third most abundant element (after oxygen and silicon) in the Earth's crust, and the most abundant metal there. It makes up about 8% by mass of the crust, though it is less common in the mantle below."[51]
Halogens

Halogens are elements in column 7A of the periodic table. They occur as a principal component in a variety of minerals.
On the right, is an example of almost completely clear fluorite (CaF2).
Impurities can give color. Some halogen minerals are called halides.
Metalloids

Metalloids are elements whose properties are intermediate between metals and solid nonmetals or semiconductors.
A variety of elements are often considered metalloids:
- boron, considered here in the boronides,
- aluminum, a face-centered cubic metal, considered in the aluminides,
- silicon, here in the siliconides,
- gallium (it can occur in the liquid state as a mineraloid),
- germanium,
- arsenic,
- selenium, also included in the chalcogens,
- indium,
- tin,
- antimony,
- tellurium, also included in the chalcogens,
- polonium, considered as among the heavy metals and
- astatine, here is with the halogens.
Metal minerals

Metals constitute a large portion of the Periodic Table of elements. Their combinations with each other and the other elements result in the minerals that compose rocky-objects.
Generally, the transition metals constitute the periodic table of elements from groups 3-12, beginning with scandium (Sc) and ending with element number 112 Copernicium.
Usually, these and the other metals are divided into several subgroups for specific purposes:
- Metalloids - gallium (Ga) through selenium (Se) and cadmium (Cd) through tellurium (Te).
- Alkaline earth metals - group 2: beryllium (Be) through radium (Ra).
- Alkali metals - group 1: lithium (Li) through francium (Fr).
- Body-centered cubic metals - titanium (Ti) through chromium (Cr), zirconium (Zr) through molybdenum (Mo), and hafnium (Hf) through tungsten (W).
- Heavy metals - mercury (Hg) through polonium (Po).
- Lanthanides - lanthanum (La) through lutetium (Lu), also called the rare-earths.
- Precious metals - ruthenium (Ru) through silver (Ag) and rhenium (Re) through gold (Au).
- Siliconides.
- Transition metals are often restricted to manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
- Transuranics - neptunium (Np) through Roentgenium (Rg) and Copernicium (Cn).
The category of metals also includes aluminum, scandium (Sc), yttrium (Y), technetium (Tc), and the actinides.
Alkaline earth metal minerals

Bromellite is BeO, with 50 at % beryllium.[5]
Alkali metal minerals
USGOV.jpg)
The alkalis, or alkali metals, are the group 1 elements of the Periodic Table. In addition to the true metals: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr), hydrogen (H) is usually included.
Each of the elements in group 1 has or may have native mineral occurrences on Earth or elsewhere nearby.
Halite (NaCl) is probably the most common mineral containing sodium at 50 at %. It is a cubic mineral usually found in arid locations on Earth. Occurrences have clear, white, purple, blue, yellow, orange, and red varieties.[5]
Body-centered cubic metal minerals
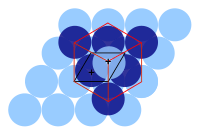
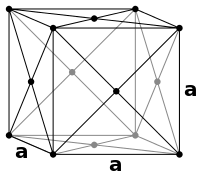
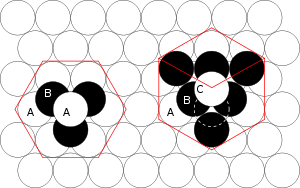

Metals in general fall into two groups of close-packing structures: face-centered cubic (fcc) and hexagonal close-packed (hcp). These two are the most efficient way to pack a bunch of hard marbles into the smallest space.
An fcc structure differs from a hcp in the number of distinct close-packed layers: hcp has two, symbolized with A below B, and fcc which has three with A below B, but C above B before A again.
Native chromium such as the nugget in the image on the right is very rare. It is also a hard mineral, probably because of an oxide coating giving it a slight bluish cast.
Precious metal minerals

Precious metals are usually rare, chemically relatively inert, and often colorful.
They are transition metals ruthenium (Ru) through silver (Ag) and rhenium (Re) through gold (Au).
Transition metal minerals

Usually, these and the other metals are divided into several subgroups for specific purposes:
- Actinides - group 3: actinium (Ac) through lawrencium (Lr).
- Alkali metals - group 1: lithium (Li) through francium (Fr).
- Alkaline earth metals - group 2: beryllium (Be) through radium (Ra).
- Aluminides - aluminum (Al): native aluminum and minerals above 25 at % Al.
- Body-centered cubic metals - titanium (Ti) through chromium (Cr), zirconium (Zr) through molybdenum (Mo), and hafnium (Hf) through tungsten (W).
- Heavy metals - mercury (Hg) through polonium (Po), copernicium (Cn) through livermorium (Lv).
- Lanthanides - lanthanum (La) through lutetium (Lu), also called the rare-earths.
- Metalloids - gallium (Ga) through selenium (Se) and cadmium (Cd) through tellurium (Te).
- Precious metals - technetium (Tc) through silver (Ag) and rhenium (Re) through gold (Au).
- Rare earth metals group 3: scandium (Sc), yttrium (Y), the Lanthanides, and the Actinides.
- Siliconides - native silicon and minerals above 25 at % Si.
- Transition metals are often restricted to manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn), but generally include Sc through zinc (Zn), Y through cadmium (Cd), lanthanum through mercury, actinium through copernicium (Cn).
- Transuranics - neptunium (Np) through roentgenium (Rg) and copernicium (Cn).
For this lecture, the transition metals are limited to those minerals containing significant quantities of manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
Generally, a mineral is a transition metal mineral if it is more than 25 at % transition metals.
Kamacites

"Kamacite is an alloy of iron and nickel, which is only found on earth in meteorites. The proportion iron:nickel is between 90:10 to 95:5; small quantities of other elements, such as cobalt or carbon may also be present. The mineral has a metallic luster, is gray and has no clear cleavage although the structure is isometric-hexoctahedral. Its density is around 8 g/cm³ and its hardness is 4 on the Mohs scale. It is also sometimes called balkeneisen."[52]
Taenites
"Taenite (Fe,Ni) is a mineral found naturally on Earth mostly in iron meteorites. It is an alloy of iron and nickel, with[nickel proportions of 20% up to 65%. ... Taenite is one of four known Fe-Ni meteorite minerals: The others are kamacite, tetrataenite, and antitaenite. ... It is opaque with a metallic grayish to white color. The structure is isometric-hexoctahedral. Its density is around 8 g/cm³ and hardness is 5 to 5.5 on the Mohs scale. Taenite is magnetic. The crystal lattice has the c≈a= 3.582ű0.002Å.[53] The Strunz classification is I/A.08-20, while the Dana classification is 1.1.11.2 . It is a Hexoctahedral (cubic) in structure."[54]
Tetrataenites
"Tetrataenite is a native metal found in meteorites with the composition FeNi. It is one of the mineral phases found in meteoric iron.[55][56][57]"[58]
Antitaenites
"Antitaenite is a meteoritic metal alloy mineral composed of iron and nickel, 20-40% Ni (and traces of other elements) that has a face centered cubic crystal structure. ... [It exists] as a new mineral species occurring in both iron meteorites and in chondrites[59] ... The pair of minerals antitaenite and taenite constitute the first example in nature of two minerals that have the same crystal structure (face centered cubic) and can have the same chemical composition (same proportions of Fe and Ni) - but differ in their electronic structures: taenite has a high magnetic moment whereas antitaenite has a low magnetic moment.[60] [This difference] arises from a high-magnetic-moment to low-magnetic-moment transition occurring in the Fe-Ni bi-metallic alloy series.[61]"[62]
Covellites

"[C]ovellite has been found in veins at depths of 1,150 meters, as the primary mineral. Covellite formed as clusters in these veins reaching one meter across".[63]
Oxidanes

Oxidane minerals contain more than 25 at or molecular % H2O.
The most common oxidane on the surface of the Earth is the liquid known as water. It occurs in the atmosphere as water vapor, and as a mineral usually referred to as ice.
Ices

"A megacryometeor is a very large chunk of ice ... sometimes called huge hailstones, but do not need to form in thunderstorms."[64]
"A megacryometeor is a very large chunk of ice which, despite sharing many textural, hydro-chemical and isotopic features detected in large hailstones, is formed under unusual atmospheric conditions which clearly differ from those of the cumulonimbus cloud scenario (i.e. clear-sky conditions). They are sometimes called huge hailstones, but do not need to form in thunderstorms. Jesus Martinez-Frias, a planetary geologist at the Center for Astrobiology in Madrid, pioneered research into megacryometeors in January 2000 after ice chunks weighing up to 6.6 pounds (3.0 kg) rained on Spain out of cloudless skies for ten days."[64]
Def. "pieces of ice falling as precipitation"[65] are called hail.
Def. "[a] single ball of hail"[66] is called a hailstone.
Def. water ice crystals falling as light white flakes are called snow.
Blue ices
.jpg)
"Blue ice occurs when snow falls on a glacier, is compressed, and becomes part of a glacier ... blue ice was observed in Tasman Glacier, New Zealand in January 2011.[67] ... ice is blue for the same reason water is blue: it is a result of an overtone of an oxygen-hydrogen (O-H) bond stretch in water which absorbs light at the red end of the visible spectrum.[68]"[69]
Actinide minerals
Actinide minerals, or actinides, are those with unusually high concentrations, atomic per cents, or weight per cents, of the actinide elements.
Autunites
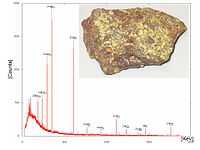
Elements usually emit a gamma-ray during nuclear decay or fission. The gamma-ray spectrum at right shows typical peaks for 226Ra, 214Pb, and 214Bi. These isotopes are part of the uranium-radium decay line. As 238U is an alpha-ray emitter, it is not shown. The peak at 40 keV is not from the mineral. From the color of the rock shown the yellowish mineral is likely to be autunite.
Autunite "occurs as [an] oxidizing product of uranium minerals in granite pegmatites and hydrothermal deposits."[70]
Pitchblendes

"Uraninite is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2, but also contains UO3 and oxides of lead, thorium, and rare earth elements. It is most commonly known as pitchblende (from pitch, because of its black color ... All uraninite minerals contain a small amount of radium as a radioactive decay product of uranium. Uraninite also always contains small amounts of the lead isotopes 206Pb and 207Pb, the end products of the decay series of the uranium isotopes 238U and 235U respectively. ... The extremely rare element technetium can be found in uraninite in very small quantities (about 0.2 ng/kg), produced by the spontaneous fission of uranium-238."[71]
The image at left shows well-formed crystals of uraninite. The image at right shows botryoidal unraninite. Because of the uranium decay products, both sources are gamma-ray emitters.
Thorianites

"Thorianite is a rare thorium oxide mineral, ThO2.[72] ... [It has a] high percentage of thorium; it also contains the oxides of uranium, lanthanum, cerium, praseodymium and neodymium. ... the mineral is slightly less radioactive than pitchblende, but is harder to shield due to its high energy gamma rays. It is common in the alluvial gem-gravels of Sri Lanka, where it occurs mostly as water worn, small, heavy, black, cubic crystals."[73]
Torbernites

"Torbernite ... is a radioactive, hydrated green copper uranyl phosphate mineral, found in granites and other uranium-bearing deposits as a secondary mineral. Torbernite is isostructural with the related uranium mineral, autunite. The chemical formula of torbenite is similar to that of autunite in which a Cu2+ cation replaces a Ca2+. The number of water hydration molecules can vary between 12 and 8, giving rise to the variety of metatorbernite when torbernite spontaneously dehydrates."[74]
Uranophanes

"Uranophane Ca(UO2)2(SiO3OH)2·5H2O is a rare calcium uranium [nesosilicate] hydrate mineral that forms from the oxidation of uranium bearing minerals. Uranophane is also known as uranotile. It has a yellow color and is radioactive."[75]
Transuranic minerals

Minerals containing transuranics are not rare. But, the stability of such isotopes makes it unlikely to find large concentrations of specific elements.
The transuranics start after uranium (U) in the periodic table. They include the named elements: plutonium (Pu), americium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No), lawrencium (Lr), rutherfordium (Rf), dubnium (Db), seaborgium (Sg), bohrium (Bh), hassium (Hs), meitnerium (Mt), darmstadtium (Ds), roentgenium (Rg), copernicium (Cn), flerovium (Fl), and livermorium (Lv).
Chalcogens

The chalcogens are the elements of group 16 of the Periodic Table. These include oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv).
Chalcogen minerals are more than 25 at % chalcogens.
Hibonites


Usually, "Hibonite ((Ca,Ce)(Al,Ti,Mg)12O19) [as shown at right] is a brownish black mineral ... It is rare, but is found in high-grade metamorphic rocks on Madagascar. Some presolar grains in primitive meteorites consist of hibonite. Hibonite also is a common mineral in the Ca-Al-rich inclusions (CAIs) found in some chondritic meteorites. Hibonite is closely related to hibonite-Fe (IMA 2009-027, ((Fe,Mg)Al12O19)) an alteration mineral from the Allende meteorite.[76] [Hibonite] is blue [perhaps like the image at left] in meteorite occurrence."[77]
Carbonates

Carbonates have more than 25 at or molecular % CO3.
Calcite has the chemical formula CaCO3.[5]
Calcite contains one oxide: CO3, or carbonate. It is 50 molecular % carbonate and 50 at % calcium.
Malachites

Malachite is a mineral that occurs in rocks at or near the interface between Earth's atmosphere and crust.
Carbonide minerals

Carbonides are naturally occurring minerals composed of 50 atomic percent, or more, carbon. Carbonide-like minerals with greater than 25 at % carbon are also included. This separates carbon containing minerals from carbonates which are at most 25 at % carbon.
Diamonds

Def. "[a] naturally occurring, glimmering glass-like allotrope of carbon in which each atom is surrounded by four others in the form of a tetrahedron"[79] is called a diamond.
Carpathites


Def. a solid, homogeneous, monoclinic (space group P2/c, no. 13, or P21/c, no. 14), naturally occurring, chemical compound with the formula C24H12 that results from natural inorganic processes is called a carpathite.
Carpathite (aka Karpatite) is a very rare organic species (C24H12).[5] It is a polycyclic aromatic hydrocarbon (PAH). This specimen is from the old Picacho Mercury Mine of California. It exhibits a radial spray of highly lustrous, canary-yellow carpathite lathes to 2.0 cm on drusy quartz. Another crossed cluster of crystals above reach 3.0 cm. It has 66.7 at % carbon.
Explorations
"Airborne gamma-ray spectrometry is now the accepted leading technique for uranium prospecting with worldwide applications for geological mapping, mineral exploration & environmental monitoring."[80]
Moon

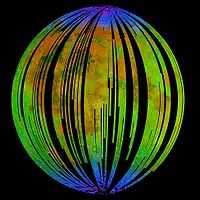
"These images show a very young lunar crater on the side of the moon that faces away from Earth, as viewed by NASA's Moon Mineralogy Mapper [M3] on the Indian Space Research Organization's Chandrayaan-1 spacecraft. On the left is an image showing brightness at shorter infrared wavelengths. On the right, the distribution of water-rich minerals (light blue) is shown around a small crater. Both water- and hydroxyl-rich materials were found to be associated with material ejected from the crater."[81]
"NASA's Moon Mineralogy Mapper, an instrument on the Indian Space Research Organization's Chandrayaan-1 mission, took [the image at right] of Earth's moon. It is a three-colour composite of reflected near-infrared radiation from the sun, and illustrates the extent to which different materials are mapped across the side of the moon that faces Earth. Small amounts of water were detected on the surface of the moon at various locations. This image illustrates their distribution at high latitudes toward the poles. Blue shows the signature of water, green shows the brightness of the surface as measured by reflected infra-red radiation from the sun and red shows a mineral called pyroxene."[82]
Europa
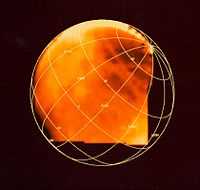
"Frozen sulfuric acid on Jupiter's moon Europa is depicted in this image produced from data gathered by NASA's Galileo spacecraft. The brightest areas, where the yellow is most intense, represent regions of high frozen sulfuric acid concentration. Sulfuric acid is found in battery acid and in Earth's acid rain."[83]
"This image is based on data gathered by Galileo's near infrared mapping spectrometer."[83]
"Europa's leading hemisphere is toward the bottom right, and there are enhanced concentrations of sulfuric acid in the trailing side of Europa (the upper left side of the image). This is the face of Europa that is struck by sulfur ions coming from Jupiter's innermost moon, Io. The long, narrow features that crisscross Europa also show sulfuric acid that may be from sulfurous material extruded in cracks."[83]
"[T]he darker regions are areas where Europa's primarily water ice surface has a higher mineral content."[84]
Enceladus

At right is a pair of images showing "[a] fine spray of small, icy particles emanating from the warm, geologically unique province surrounding the south pole of Saturn’s moon Enceladus[. It] was observed in a Cassini narrow-angle camera image of the crescent moon taken on Jan. 16, 2005. Taken from a high-phase angle of 148 degrees -- a viewing geometry in which small particles become much easier to see -- the plume of material becomes more apparent in images processed to enhance faint signals [right image of the pair]."[85]
"Though the measurements of particle abundance are more certain within 100 kilometers (60 miles) of the surface, the values measured there are roughly consistent with the abundance of water ice particles measured by other Cassini instruments (reported in September, 2005) at altitudes as high as 400 kilometers (250 miles) above the surface."[85]
"The image at the left was taken in visible green light. A dark mask was applied to the moon's bright limb in order to make the plume feature easier to see."[85]
"The image at the right has been color-coded to make faint signals in the plume more apparent. Images of other satellites (such as Tethys and Mimas) taken in the last 10 months from similar lighting and viewing geometries, and with identical camera parameters as this one, were closely examined to demonstrate that the plume towering above Enceladus' south pole is real and not a camera artifact. The images were acquired at a distance of about 209,400 kilometers (130,100 miles) from Enceladus. Image scale is about 1 kilometer (0.6 mile) per pixel."[85]
Neptune
.tif.jpg)
The set of four images at right are taken with the Hubble Space Telescope on June 25-26, 2011, just before Neptune arrived at the same location in space where it was discovered nearly 165 (Earth) years before (i.e. 1 Neptunian year on July 11, 2011).
"The snapshots were taken at roughly four-hour intervals [of a 16 h rotation period], offering a full view of the planet. The images reveal high-altitude clouds in the northern and southern hemispheres. The clouds are composed of methane ice crystals."[86]
"The snapshots show that Neptune has more clouds than a few years ago, when most of the clouds were in the southern hemisphere. These Hubble views reveal that the cloud activity is shifting to the northern hemisphere. It is early summer in the southern hemisphere and winter in the northern hemisphere."[86]
"The clouds are tinted pink because they are reflecting near-infrared light."[86]
Petrology


Def. "the study of the origin, composition and structure of rock"[87] is called petrology.
Def. "[a] section formed by a plane cutting through an object, usually at right angles to an axis"[88] is called a cross section.
Def. "a laboratory preparation of a rock, mineral, soil, pottery, bones, or metal sample for use with a polarizing petrographic microscope, electron microscope and electron microprobe"[89] is called a thin section.
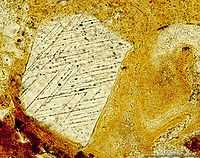
At lower left is a thin section through a sand-sized quartz grain "from the USGS-NASA Langley core showing two well-developed, intersecting sets of shock lamellae produced by the late Eocene Chesapeake Bay bolide impact. This shocked quartz grain is from the upper part of the crater-fill deposits at a depth of 820.6 ft in the core. The corehole is located at the NASA Langley Research Center, Hampton, VA, near the southwestern margin of the Chesapeake Bay impact crater."[90] "Very high pressures produced by strong shock waves cause dislocations in the crystal structure of quartz grains along preferred orientations. These dislocations appear as sets of parallel lamellae in the quartz when viewed with a petrographic microscope. Bolide impacts are the only natural process known to produce shock lamellae in quartz grains."[90]
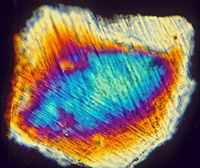
Lower right shows another thin section in plane polarized light of a shocked quartz grain with two sets of decorated planar deformation features (PDFs) surrounded by a cryptocrystalline matrix from the Suvasvesi South impact structure, Finland.
In a specimen of shocked quartz, "stishovite can be separated from quartz by applying hydrogen fluoride (HF); unlike quartz, stishovite will not react.[91]"[16]
Petrography
Def. "the scientific description and classification of rocks"[92] is called petrography.
Original research
- See also: Original research inquiry and Research
Hypothesis:
- High-temperature, high-pressure minerals exist in the core of the Earth.
- High-temperature, high-pressure minerals can be produce through natural electrochemistry.
- See also: Control groups, Proof of concept, and Proof of technology
See also
References
- ↑ Dmh (6 September 2004). "material, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-11-05.
- ↑ "mineral, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 29, 2013. Retrieved 2013-08-30.
- ↑ "metasilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 20, 2013. Retrieved 2013-09-02.
- ↑ "neosilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 15, 2013. Retrieved 2013-09-02.
- 1 2 3 4 5 6 7 8 Willard Lincoln Roberts, George Robert Rapp, Jr., and Julius Weber (1974). Encyclopedia of Minerals. 450 West 33rd Street, New York, New York 10001 USA: Van Nostrand Reinhold Company. pp. 693. ISBN 0-442-26820-3.
- 1 2 "orthosilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 16, 2013. Retrieved 2013-09-02.
- ↑ "sorosilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 19, 2013. Retrieved 2013-09-02.
- ↑ "inosilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 21 June 2013. Retrieved 2013-09-02.
- ↑ "phyllosilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 17, 2013. Retrieved 2013-09-02.
- ↑ "tectosilicate, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. June 17, 2013. Retrieved 2013-09-02.
- ↑ "Quartz, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 22, 2012. Retrieved 2012-10-23.
- 1 2 "Shocked quartz, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 14, 2012. Retrieved 2012-10-23.
- ↑ "Seifertite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. December 5, 2011. Retrieved 2012-10-23.
- ↑ "Tridymite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 25, 2012. Retrieved 2012-10-23.
- ↑ "coesite, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 3, 2012. Retrieved 2012-10-23.
- 1 2 3 "Stishovite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 24, 2012. Retrieved 2012-10-23.
- ↑ R Wirth, C. Vollmer, F. Brenker, S. Matsyuk, F. Kaminsky (2007). "Inclusions of nanocrystalline hydrous aluminium silicate "Phase Egg" in superdeep diamonds from Juina (Mato Grosso State, Brazil)". Earth and Planetary Science Letters 259 (3–4): 384. doi:10.1016/j.epsl.2007.04.041.
- ↑ "Cristobalite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 26, 2012. Retrieved 2012-10-23.
- ↑ "Sodalite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 25, 2013. Retrieved 2013-05-28.
- ↑ "Lazurite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 21, 2013. Retrieved 2013-05-28.
- ↑ http://rruff.geo.arizona.edu/doclib/hom/glaucophane.pdf Handbook of Mineralogy
- ↑ "Glaucophane, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. March 4, 2013. Retrieved 2013-05-28.
- ↑ Farndon and Parker (2009). Minerals, Rocks and Fossils of the World. Lorenz Books
- ↑ http://rruff.info/ima
- 1 2 3 Gaines et al (1997). Dana’s New Mineralogy Eighth Edition. New York: Wiley.
- 1 2 "Hauyne". Mindat.org. Retrieved 08/11/11.
- ↑ "Hauyne". Webminerals. Retrieved 08/11/11.
- ↑ Handbook of Mineralogy
- ↑ "Hauyne, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 25, 2013. Retrieved 2013-05-28.
- ↑ "olivine, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 30, 2012. Retrieved 2012-10-23.
- 1 2 3 "Forsterite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 24, 2012. Retrieved 2013-01-22.
- ↑ Ds. Lauretta, L.P. Keller, S. Messenger (2005). "Supernova olivine from cometary dust". Science 309 (5735): 737–741. doi:10.1126/science.1109602. PMID 15994379.
- ↑ Spitzer sees crystal 'rain' in outer clouds of infant star, Whitney Clavin and Trent Perrotto, Physorg.com, May 27, 2011. Accessed May 2011
- ↑ "Greenstone belt, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. January 10, 2013. Retrieved 2013-01-22.
- ↑ "Pyroxene, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 21, 2013. Retrieved 2013-09-01.
- ↑ Deer, W. A., R. A. Howie and J. Zussman (1966) An Introduction to the Rock Forming Minerals, Longman, ISBN 0-582-44210-9
- ↑ "feldspar, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. October 16, 2012. Retrieved 2012-10-23.
- ↑ "plagioclase, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. October 16, 2012. Retrieved 2012-10-23.
- ↑ "mineraloid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. April 20, 2011. Retrieved 2012-10-23.
- ↑ "amber, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. October 18, 2012. Retrieved 2012-10-23.
- ↑ "petroleum, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 10, 2012. Retrieved 2012-10-23.
- ↑ "tektite, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 31, 2012. Retrieved 2012-10-23.
- ↑ "Opal, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 19, 2012. Retrieved 2012-10-23.
- 1 2 "Moldavite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. January 21, 2013. Retrieved 2013-01-25.
- ↑ Trnka, M.; Houzar, S. (2002). "Moldavites: a review PDF". Bulletin of the Czech Geological Survey 77 (4): 283–302. http://www.geology.cz/bulletin/contents/2002/vol77no4/04trnkafinal.pdf.
- ↑ Serefiddin, F.; Herzog, G. F.; Koeberl, C. (2007). "Beryllium-10 concentrations of tektites from the Ivory Coast and from Central Europe: Evidence for near-surface residence of precursor materials". Geochimica et Cosmochimica Acta 71 (6): 1574–82. http://www.univie.ac.at/geochemistry/koeberl/publikation_list/296-Be-10-Ivory-Coast-tektites-and-moldavites-GCA2007.pdf.
- 1 2 "rock, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. October 23, 2012. Retrieved 2012-10-23.
- ↑ "rocky, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 29, 2012. Retrieved 2012-10-23.
- ↑ Philip B. Gove, ed (1963). Webster's Seventh New Collegiate Dictionary. Springfield, Massachusetts: G. & C. Merriam Company. pp. 1221.
- ↑ "crystallography, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 29, 2013. Retrieved 2013-09-02.
- ↑ "Aluminium, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. 28 October 2015. Retrieved 2015-10-28.
- ↑ "Kamacite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 4, 2013. Retrieved 2013-09-01.
- ↑ Albertsen, F.; Knudsen, J. M.; Jensen, G. B. (Jun 1978). "Structure of taenite in two iron meteorites J.". Nature 273 (5662): 453–454. doi:10.1038/273453a0.
- ↑ "Taenite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 27, 2013. Retrieved 2013-09-01.
- ↑ "Tetrataenite". webmineral.com.
- ↑ Mindat.org - Tetrataenite
- ↑ Handbook of Mineralogy - Tetrataenite
- ↑ "Tetrataenite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 24, 2013. Retrieved 2013-09-01.
- ↑ D.G. Rancourt and R.B. Scorzelli. Low Spin γ-Fe-Ni (γLS) Proposed as a New Mineral in Fe-Ni-Bearing Meteorites: Epitaxial Intergrowth of γLS and Tetrataenite as Possible Equilibrium State at ~20-40 at % Ni. Journal of Magnetism and Magnetic Materials 150 (1995) 30-36
- ↑ D.G. Rancourt, K. Lagarec, A. Densmore, R.A. Dunlap, J.I. Goldstein, R.J. Reisener, and R.B. Scorzelli. Experimental Proof of the Distinct Electronic Structure of a New Meteoritic Fe-Ni Alloy Phase. Journal of Magnetism and Magnetic Materials 191 (1999) L255-L260
- ↑ K. Lagarec, D.G. Rancourt, S.K. Bose, B. Sanyal, and R.A. Dunlap. Observation of a composition-controlled high-moment/low-moment transition in the face centered cubic Fe-Ni system: Invar effect is an expansion, not a contraction. Journal of Magnetism and Magnetic Materials 236 (2001) 107-130.
- ↑ "Antitaenite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 7, 2013. Retrieved 2013-09-01.
- ↑ "Covellite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 25, 2013. Retrieved 2013-05-28.
- 1 2 "Megacryometeor, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 7, 2012. Retrieved 2012-10-13.
- ↑ "hail, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. February 9, 2013. Retrieved 2013-02-15.
- ↑ "hailstone, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. December 26, 2012. Retrieved 2013-02-15.
- ↑ Harvey, Eveline (14 January 2011). "NZ blue ice sighting an unexpected treat for tourists". The New Zealand Herald. Retrieved 21 September 2011.
- ↑ Why Is Water Blue
- ↑ "Blue ice (glacial), In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. March 29, 2013. Retrieved 2013-05-29.
- ↑ "Autunite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 21, 2013. Retrieved 2013-05-06.
- ↑ "Uraninite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 20, 2013. Retrieved 2013-05-08.
- ↑ C. Frondel (1958). Systematic Mineralogy of Uranium and Thorium. United States Government Printing Office.
- ↑ "Thorianite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 27, 2013. Retrieved 2013-05-08.
- ↑ "Torbernite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 27, 2013. Retrieved 2013-05-08.
- ↑ "Uranophane, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 28, 2013. Retrieved 2013-05-08.
- ↑ IMA Mineral List with Database of Mineral Properties
- ↑ "Hibonite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. March 7, 2013. Retrieved 2013-06-01.
- ↑ "Malachite". WebExhibits. 2001. Retrieved 2007-12-08.
- ↑ "diamond, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. October 16, 2012. Retrieved 2012-10-23.
- ↑ "Uranium mining, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. April 5, 2013. Retrieved 2013-05-07.
- ↑ Jim Wilson (September 24, 2009). "Water Around a Fresh Crater". NASA. Retrieved 2012-09-26.
- ↑ Yvette Smith (September 25, 2009). "Water Detected at High Latitudes on the Moon". NASA. Retrieved 2012-11-26.
- 1 2 3 Sue Lavoie (September 30, 1999). "PIA02500: Sulfuric Acid on Europa". Washington DC USA: NASA's Office of Space Science. Retrieved 2013-06-24.
- ↑ "Europa (moon), In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 22, 2013. Retrieved 2013-06-24.
- 1 2 3 4 Sue Lavoie (November 28, 2005). "PIA07760: Spray Above Enceladus". Pasadena, California: NASA and the Jet Propulsion Laboratory, California Institute of Technology. Retrieved 2012-07-22.
- 1 2 3 Donna Weaver, Ray Villard, Keith Noll (July 12, 2011). "Neptune Completes Its First Circuit Around The Sun Since Its Discovery". Baltimore, Maryland, USA: NASA/Space Telescope Science Institute. Retrieved 2012-11-26.
- ↑ "petrology, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 31, 2012. Retrieved 2012-10-23.
- ↑ "cross section, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. October 16, 2012. Retrieved 2012-10-23.
- ↑ "Thin section, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 11, 2012. Retrieved 2012-10-23.
- 1 2 Glen A. Izett (September 26, 2000). "Shocked Quartz from the USGS -- NASA Langley Core". U. S. Geological Survey. Retrieved 2012-10-23.
- ↑ Michael Fleischer (1962). "New mineral names". American Mineralogist (Mineralogical Society of America) 47 (2): 172–4. http://rruff.info/uploads/AM47_805.pdf.
- ↑ "petrography, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. December 19, 2011. Retrieved 2012-10-23.
External links
- African Journals Online
- Bing Advanced search
- GenomeNet KEGG database
- Google Books
- Google scholar Advanced Scholar Search
- Home - Gene - NCBI
- International Astronomical Union
- JSTOR
- Lycos search
- NASA/IPAC Extragalactic Database - NED
- NASA's National Space Science Data Center
- NCBI All Databases Search
- NCBI Site Search
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- Questia - The Online Library of Books and Journals
- SAGE journals online
- The SAO/NASA Astrophysics Data System
- Scirus for scientific information only advanced search
- SIMBAD Astronomical Database
- SIMBAD Web interface, Harvard alternate
- SpringerLink
- Taylor & Francis Online
- Universal coordinate converter
- WikiDoc The Living Textbook of Medicine
- Wiley Online Library Advanced Search
- Yahoo Advanced Web Search
| |||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is an astronomy resource. |
| |
Subject classification: this is a chemistry resource . |
| |
Subject classification: this is a Geology resource. |
| |
Subject classification: this is a materials science resource. |


