Liquids

A liquid is any substance in which its constituent parts stick together but do not crystallize.
The image on the right demonstrates that this substance's constituent parts are sticking together but not crystallizing as it flows from a bottle into a glass.
Theoretical liquids
Def. any substance having a consistency of flowing freely with a constant volume is called a liquid.
Def. a substance that is flowing, and keeping no shape, a substance of which the molecules, while not tending to separate from one another like those of a gas, readily change their relative position, and which therefore retains no definite shape, except that determined by the containing receptacle is called a liquid.
Metadef.
- a flowing substance
- keeping or retaining no shape or definite shape
- a. except that determined by the containing receptacle
- 3. composed of molecules
- a. not tending to separate from one another like those of a gas
- b. readily changing relative position
is called a liquid.
Clear liquids
Metadefinition of a clear liquid:
Metadef.
- a clear flowing substance
- keeping or retaining no shape or definite shape
- a. except that determined by the containing receptacle
- 3. composed of molecules
- a. not tending to separate from one another like those of a gas
- b. readily changing relative position
is called a clear liquid.
"A clear liquid diet consists of transparent liquid foods such as vegetable broth, bouillon, clear fruit juices, clear fruit ices, popsicles, clear gelatin desserts, and no carbonated drinks. Soda's carbonation expands the gastrointestinal tract."[1] Herein are many clear liquids that fit within the metadefinition.
Liquid objects
Def. any object consisting of at least 2 % by particle, or least divisible particle, number of liquids is called a liquid object.
Mineraloids
Def. a "substance that resembles a mineral but does not exhibit crystallinity"[2] is called a mineraloid.
Native liquid elements include bromines, galliums and mercuries.
Waters
Def. a clear liquid having the chemical formula H2O, required by all forms of life on Earth is called water.
Def. of water from the metadefinition of a clear liquid:
- a clear flowing substance
- keeping or retaining no shape or definite shape
- a. except that determined by the containing receptacle
- 3. composed of H2O molecules
- a. not tending to separate from one another like those of a gas
- b. readily changing relative position
is called water.
Ethanols
Def. of ethanol from the metadefinition of a clear liquid:
- a clear flowing substance
- keeping or retaining no shape or definite shape
- a. except that determined by the containing receptacle
- 3. composed of C2H5OH molecules
- a. not tending to separate from one another like those of a gas
- b. readily changing relative position
is called ethanol.
Limonites

"Limonite is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·nH2O, although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Limonite is one of the two principle iron ores, the other being hematite, and has been mined for the production of iron since at least 2500 BCE.[3][4] ... Although originally defined as a single mineral, limonite is now recognized as a mixture of related hydrated iron oxide minerals, among them goethite, akaganeite, lepidocrocite, and jarosite. Individual minerals in limonite may form crystals, but limonite does not, although specimens may show a fibrous or microcrystalline structure,[5] and limonite often occurs in concretionary forms or in compact and earthy masses; sometimes mammillary, botryoidal, reniform or stalactitic. Because of its amorphous nature, and occurrence in hydrated areas limonite often presents as a clay or mudstone. However there are limonite pseudomorphs after other minerals such as pyrite.[6] This means that chemical weathering transforms the crystals of pyrite into limonite by hydrating the molecules, but the external shape of the pyrite crystal remains. Limonite pseudomorphs have also been formed from other iron oxides, hematite and magnetite; from the carbonate siderite and from iron rich silicates such as almandine garnets. ... Limonite usually forms from the hydration of hematite and magnetite, from the oxidation and hydration of iron rich sulfide minerals, and chemical weathering of other iron rich minerals such as olivine, pyroxene, amphibole, and biotite. It is often the major iron component in lateritic soils. ... One of the first uses was as a pigment. The yellow form produced yellow ochre for which Cyprus was famous,[7]."[8]
Petroleums
Def. a "flammable liquid ranging in color from clear to very dark brown and black, consisting mainly of hydrocarbons, occurring naturally in deposits under the Earth's surface"[9] is called a petroleum.
Coal tars

Def. a "black, oily, sticky, viscous substance, consisting mainly of hydrocarbons derived from organic materials such as wood, peat, or coal"[10] is called a tar.
Def. a thick black liquid produced by the destructive distillation of bituminous coal is called a coal tar.
It contains at least benzene, naphthalene, phenols, and aniline.
Naphthas

Def. any "of a wide variety of aliphatic or aromatic liquid hydrocarbon mixtures distilled from petroleum or coal tar"[11] is called a naphtha.
Malthas
Def. a black viscid substance intermediate between petroleum and asphalt is called a maltha, or malthite.
Bitumens
Def. a black viscous mixture of hydrocarbons obtained naturally is called a bitumen.
In the image on the right, bitumen occurs with lussatite, an opal.
Pitchs


Def. a "dark, extremely viscous material remaining in still after distilling crude oil and tar"[12] is called a pitch.
Asphalts

Def. a "sticky, black and highly viscous liquid or semi-solid, composed almost entirely of bitumen, that is present in most crude petroleums and in some natural deposits"[13] is called an asphalt.
Zietrisikites
Def. a natural, waxy hydrocarbon mineraloid is called a zietrisikite.
Ozocerites

Def. a natural dark, or black, odoriferous mineraloid wax is called ozokerite, or ozocerite.
Ambers


Def. a "hard, generally yellow to brown translucent fossil resin"[14] is called an amber.
Hydrogens


The image on the right shows that liquid hydrogen can be poured.
The second image down on the right shows a liquid hydrogen bubble chamber used to detect subatomic particles from a bevatron.
Heliums

Liquid helium as shown on the right is just above absolute zero in temperature.
Nitrogens

On the right liquid nitrogen is shown being poured.
Oxygens

Liquid oxygen as shown on the right may not be perfectly clear.
"Liquid oxygen has a pale blue color and is strongly paramagnetic and can be suspended between the poles of a powerful horseshoe magnet. Liquid oxygen has a density of 1.141 kg/L and is cryogenic. [F]reezing point: 50.5 K (-368.77 °F; -222.65 °C), boiling point: 90.19 K (-297.33 °F, -182.96 °C) at 101.325 kPa (760 mmHg)."[15]
Fluorines

Liquid fluorine in the tube on the right is yellow-orange in color.
Sulfurs

At volcanic locations, native liquid sulfur can be seen flowing, such as in the image on the right, and it is red.
Chlorines


On the right is liquid chlorine (Cl2) contained in a flask for analysis. Liquid chlorine is yellow in color.
Image on the left is an ampoule containing liquid chlorine. It has been liquefied under pressure at >7.4 bar, sealed in the quartz vial (ampoule), further sealed in an "acrylic glass" (i.e., Plexiglas, Lucite) cube, cube edge length: 5 cm.
Argons

While argon is a gas at room temperature and pressure, it becomes a solid at liquid nitrogen temperature and melts to a liquid as in the image on the right when removed from the liquid nitrogen.
Galliums

The second image down on the right is a drop of liquid gallium.
Bromines
.png)
Bromine is a halogen that is a liquid at room temperature and pressure.
Indiums

Indium has a low melting temperature of 156.6°C.
Mercuries

The metallic element mercury is a liquid at room temperature and pressure as shown in the image on the right.
Def. a naturally occurring, silvery-colored, metallic liquid, composed primarily of the chemical element mercury, is called mercury, or native mercury.
Rocks





Def. "melted rock ejected by a volcano from its crater or fissured sides"[16] is called a lava.
Usage notes
"Geologists make a distinction between magma (molten rock underground) and lava (molten rock on the surface)."[16]
Def. "molten matter within the earth, the source of the material of lava flows, dikes of eruptive rocks, etc"[17] is called a magma.
"Streams of molten rock that ooze from gaps or vents in the Earth’s surface are called lava flows. Though generally slow-moving, these rivers of rock pose a hazard to everything in their paths. They can bury or burn homes and roads, ruin farmland for generations, and transform glaciers into muddy landslides (lahars)."[18]
"Lava flows can take many shapes and move at very different rates depending on the viscosity of the magma, the slope of the land, and the rate of an eruption. Some of the speediest flows travel 60 kilometers (40 miles) per hour; the slowest creep along at less than 1 kilometer (0.6 miles) per hour. They can sometimes even flow for more than a year after an eruption has ended."[18]
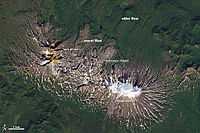
"While viscous lava flows are defined by steep flow fronts and pressure ridges, low-viscosity lavas tend to move faster and create longer, narrower shapes. They also tend to have smaller flow fronts and levee-like structure along their edges. Many characteristics of a low-viscosity lava flow are visible in [the image on the right second down] of Zhupanovsky and Dzenzursky volcanoes on Russia’s Kamchatka Peninsula. The image was acquired by the Operational Land Imager (OLI) on the Landsat 8 satellite on September 9, 2013."[19]
"In the image, younger lava flows appear grey, while older flows are covered by green vegetation. The exact ages of the flows are unclear, but the eruptions that produced them likely occurred during the past few thousand years. Distinctive lava levees are visible along the edges of many of the younger flows. These features form as lava cools and hardens along the edges or top of a flow while the center of a flow still advances."[19]
"In comparison to the Chao dacite in Chile (the product of viscous lava), the flows at Zhupanovsky [at lowest right] and Dzenzursky are much narrower and longer. They have smaller flow fronts (10 to 30 meters tall) in comparison to the sheer 400-meter cliffs at Chao, as well as more prominent lava levees along the edges. Like Chao, the flows shown above have pressure ridges caused by the compression of the cooling top of the lava as the flow advanced."[19]
Def. a "form of lava flow of basaltic rock, usually dark-colored with a smooth or ropey surface"[20] is called pahoehoe.
"Ropy pahoehoe [shown in the fourth image down on the right, taken 11 June 1995] is the most common surface texture of pahoehoe flows. The numerous folds and wrinkles ("ropes") that are characteristic of ropy pahoehoe form when the thin, partially solidified crust of a flow is slowed or halted (for example, if the crust encounters an obstruction or slower-moving crust). Because lava beneath the crust continues to move forward, it tends to drag the crust along. The crust then behaves like an accordian that is squeezed together--the crust is flexible enough to develop wrinkles or a series of small ridges and troughs as it is compressed and driven forward."[21]
Def. a "form of lava flow [shown on the right, fifth image down] consisting of basaltic rock, usually dark-colored with a jagged and loose, clinkery surface"[22] is called an aa.
"`A`a (pronounced "ah-ah") is a Hawaiian term for lava flows that have a rough rubbly surface composed of broken lava blocks called clinkers. The incredibly spiny surface of a solidified `a`a flow makes walking very difficult and slow. The clinkery surface actually covers a massive dense core, which is the most active part of the flow. As pasty lava in the core travels downslope, the clinkers are carried along at the surface. At the leading edge of an `a`a flow, however, these cooled fragments tumble down the steep front and are buried by the advancing flow. This produces a layer of lava fragments both at the bottom and top of an `a`a flow."[23]
Obsidians

An example of obsidian is shown on the right. Obsidian is a naturally occurring glass. Glass is an extremely viscous liquid.
Def. a naturally occurring black glass is called an obsidian.
Tektites
Def. "[a] small, round, dark glassy object, composed of silicates"[24] is called a tektite.
Opals
Def. a naturally occurring, hydrous, "amorphous form of silica, ... [where] 3% to 21% of the total weight is water"[25] is called an opal.
Craters

In the image on the right impact from a water drop to contained but open water causes an upward "rebound" jet surrounded by circular capillary waves.
Technology
Estimated 2013 U.S. fractional helium use by category. Total use is 47 million cubic meters.[26]
Research
Hypothesis:
- Many liquids exist within specific ranges of temperature and pressure.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[27] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[28]"[29]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[30] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[31]
See also
References
- ↑ "Liquid diet, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. November 13, 2012. Retrieved 2012-12-10.
- ↑ "mineraloid, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. April 20, 2011. Retrieved 2012-10-23.
- ↑ MacEachern, Scott (1996) "Iron Age beginnings north of the Mandara Mountains, Cameroon and Nigeria" pp. 489–496 In Pwiti, Gilbert and Soper, Robert (editors) (1996) Aspects of African Archaeology: Proceedings of the Tenth Pan-African Congress University of Zimbabwe Press, Harare, Zimbabwe, ISBN 978-0-908307-55-5; archived here by Internet Archive on 11 March 2012
- ↑ Diop-Maes, Louise Marie (1996) "La question de l'Âge du fer en Afrique" ("The question of the Iron Age in Africa") Ankh 4/5: pp. 278–303, in French; archived here by Internet Archive on 25 January 2008
- ↑ Boswell, P. F. and Blanchard, Roland (1929) "Cellular structure in limonite" Economic Geology 24(8): pp. 791–796
- ↑ Northrop, Stuart A. (1959) "Limonite" Minerals of New Mexico (revised edition) University of New Mexico Press, Albuquerque, New Mexico, pp. 329–333 }}
- ↑ Constantinou, G. and Govett, G. J. S. (1972) "Genesis of sulphide deposits, ochre and umber of Cyprus" Transactions of the Institution of Mining and Metallurgy" 81: pp. 34–46
- ↑ "Limonite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. 16 September 2013. Retrieved 2013-09-16.
- ↑ "petroleum, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 16 July 2014. Retrieved 2015-01-09.
- ↑ "tar, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 5 January 2015. Retrieved 2015-01-09.
- ↑ "naphtha, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 16 December 2014. Retrieved 2015-01-09.
- ↑ "pitch, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 12 December 2014. Retrieved 2015-01-10.
- ↑ "asphalt, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 20 October 2014. Retrieved 2015-01-09.
- ↑ "amber, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 19 December 2014. Retrieved 2015-01-09.
- ↑ Vimal Cylinder Supplier (2014). "Our Products : Liquid Oxygen". 30, Shilpi Appartment, 5th Floor, Kalanala, Bhavnagar-2, Gujarat: Vimal Cylinder Supplier. Retrieved 2016-03-12.
- 1 2 "lava, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 25 January 2011. Retrieved 2015-02-17.
- ↑ "magma, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 29 December 2014. Retrieved 2015-02-17.
- 1 2 Erik Klemetti and Adam Voiland (21 November 2013). "The Shapes that Lavas Take, Part 1". Washington, DC USA: NASA. Retrieved 2015-02-18.
- 1 2 3 Erik Klemetti and Adam Voiland (22 November 2013). "The Shapes that Lavas Take, Part 2". Washington, DC USA: NASA. Retrieved 2015-02-18.
- ↑ "pahoehoe, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 31 July 2014. Retrieved 2015-02-18.
- ↑ Tari Noelani Mattox (04 September 2000). "Photo glossary of volcano terms". Menlo Park, California, USA: U.S. Geological Survey. Retrieved 2015-02-18.
- ↑ "aa, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 16 January 2015. Retrieved 2015-02-19.
- ↑ United States Geological Survey (17 July 2008). "VHP Photo Glossary: AA". Menlo Park, California USA: USGS. Retrieved 2015-03-10.
- ↑ "tektite, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. August 31, 2012. Retrieved 2012-10-23.
- ↑ "Opal, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. October 19, 2012. Retrieved 2012-10-23.
- ↑ U.S. Department of the Interior, U.S. Geological Survey (2014). "Helium". Mineral Commodity Summaries 2014. pp. 72–73. http://minerals.usgs.gov/minerals/pubs/commodity/helium/mcs-2014-heliu.pdf.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
| |||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a Geology resource. |
| |
Subject classification: this is a chemistry resource . |
| |
Subject classification: this is a physics resource . |
| |
Subject classification: this is an astronomy resource. |
