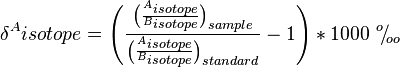Geochemistry
Geochemistry is the study of the chemical composition of the Earth and its rocks, minerals, and liquids.
Sciences
"Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans.[1] The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System[2] and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt.[1]"[3]
Theoretical geochemistry
Def. the "branch of chemistry that deals with the chemical composition of the Earth and other planets, and with the chemical processes that occur in the formation of rocks and minerals etc"[4] is called geochemistry.
Liquid objects
"Aqueous geochemistry studies the role of various elements in watersheds, including copper, sulfur, mercury, and how elemental fluxes are exchanged through atmospheric-terrestrial-aquatic interactions."[3]
Berylliums
"B/La is highly correlated with 10Be/9Be (r2 = 0.94, excluding one sample) and appears to be a useful indicator of subduction contributions to the magma sources."[5]
Borons
"Boron contents [have been] measured in representative Quaternary lavas from the Central American Volcanic Arc to evaluate along-strike variations in subduction processes."[5]
"Despite the significant range in B concentrations (~2–37 ppm) in the mafic lavas, B/La ratios vary in a systematic fashion along the arc; higher values (> 1) are typical between Guatemala and northern Costa Rica, whereas low values (most <0.5) typify central Costa Rica and western Panama."[5]
"Boron contents are uniformly low in more than 100 granulites from exposed terranes in India, Norway, and Scotland and from xenolith suites in the western USA."[6]
"Boron is apparently depleted in all granulite protoliths during prograde metamorphism and dehydration."[6]
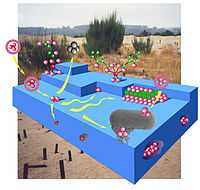
Biogeochemistry
"Biogeochemistry is the field of study focusing on the effect of life on the chemistry of the earth."[3]
Cosmochemistry

"Cosmochemistry includes the analysis of the distribution of elements and their isotopes in the cosmos."[3]
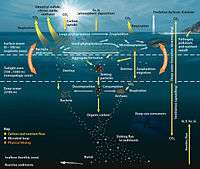
Environmental geochemistry

"[C]hemical erosion rates are greatly influenced by the yield of physical erosion and that the rapid production of fresh surfaces as a result of high physical erosion rates and subsequent denudation is critical to the high chemical erosion yields".[7]
Exploration geochemistry
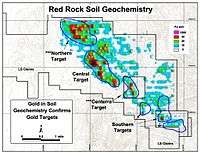
The image on the right shows the increasing presence of gold in topsoil which is correlated with gold ore deposits beneath.
Isotope geochemistry
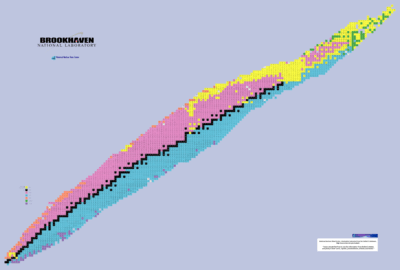
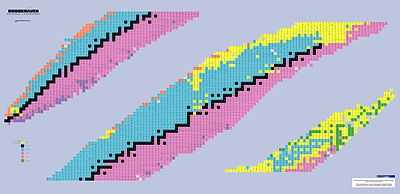
"A table of nuclides or chart of nuclides is a two-dimensional graph in which one axis represents the number of neutrons and the other represents the number of protons in an atomic nucleus. Each point plotted on the graph thus represents the nuclide of a real or hypothetical chemical element."[8] Hydrogen is at the lower left.
"Isotope geochemistry involves the determination of the relative and absolute concentrations of the [chemical] elements and their isotopes in the earth and on earth's surface."[3]
"For most stable isotopes, the magnitude of fractionation from kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand).[9]"[10]
Enrichments (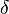 )" represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard."[10]
)" represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard."[10]
"The depletion of total [boron] B [in the Victorian volcanic-crater lakes of southeastern Australia] and the high positive δ 11B values relative to seawater (B/Cl ratio = 7.9 x 10-4; δ 11B = 39%.) are attributed to a marine (cyclic) salt origin together with adsorption processes in closed systems with low water/sediment (W/R) ratios."[11]
"Although the δ [11B] value of borate minerals may be a discriminant of marine or non-marine origin, boron isotopes are less distinctive in evaporative environments where boron is not an abundant component and where water/sediment interaction occurs."[11]
The "incidence of 18O (the heavy isotope of oxygen) can be used as an indicator of polar ice sheet extent, and boron isotopes are key indicators of the pH and CO2 content of oceans in the geologic past."[12]
"Although rubidium is monoisotopic, naturally occurring rubidium is composed of two isotopes: the stable 85Rb (72.2%) and the radioactive 87Rb (27.8%).[13] Natural rubidium is radioactive with specific activity of about 670 [Becquerel] Bq/g, enough to significantly expose a photographic film in 110 days.[14][15]"[16]
Organic geochemistry

"Organic geochemistry involves the study of the role of processes and compounds that are derived from living or once-living organisms."[3]
Regional geochemistry
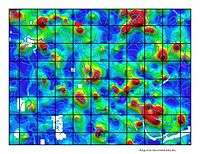
"Regional geochemistry is the study of the spatial variation in the chemical composition of materials at the surface of the Earth, on a scale of tens to thousands of kilometres. Important parameters to consider when designing or evaluating a geochemical survey are:
- Areal extent of the survey
- Sampling density
- The type of samples collected (soil, stream water, vegetation, bedrock, etc.)
- Post-collection treatment of the samples (e.g. sieving of soil samples into different particle size fractions)
- Methodology of chemical analysis"[17]
The map on the right shows a topsoil distribution of gold throughout the Quesnel region of Australia.
History
"In a word, a comparative geochemistry ought to be launched, before geochemistry can become geology, and before the mystery of the genesis of our planets and their inorganic matter may be revealed."[18]
Original research
- See also: Original research inquiry and Research
Hypothesis:
- Geochemistry as a field may be best handled as it is rather than as a subpage of geochemicals.
- See also: Control groups, Proof of concept, and Proof of technology
See also
References
- 1 2 Francis Albarède (2003). Geochemistry: An Introduction. Cambridge University Press. p. 1. ISBN 0-521-81468-5. http://books.google.co.uk/books?id=doVGzreGq14C&printsec=frontcover&hl=en&sa=X&ei=HN5fT7juGsmu8AOl05mfBw&ved=0CEIQ6AEwAA#v=onepage&f=false.
- ↑ William M. White. "Geochemistry (Unpublished)". p. 1. Retrieved 14 March 2012.
- 1 2 3 4 5 6 "Geochemistry, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. August 20, 2013. Retrieved 2013-08-29.
- ↑ SemperBlotto (November 23, 2006). "geochemistry, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2013-08-29.
- 1 2 3 William P. Leeman, Michael J. Carr, Julie D. Morris (January 1994). "Boron geochemistry of the Central American volcanic arc: Constraints on the genesis of subduction-related magmas". Geochimica et Cosmochimica Acta 58 (1): 149-68. http://www.sciencedirect.com/science/article/pii/0016703794904537. Retrieved 2013-08-29.
- 1 2 W.P. Leeman, V.B. Sisson, M.R. Reid (February 1992). "Boron geochemistry of the lower crust: evidence from granulite terranes and deep crustal xenoliths". Geochimica et Cosmochimica Acta 56 (2): 775-88. http://www.sciencedirect.com/science/article/pii/0016703792900973. Retrieved 2013-08-29.
- ↑ W. Berry Lyons, Anne E. Carey, D. Murray Hicks, and Carmen A. Nezat (March 2005). "Chemical weathering in high-sediment-yielding watersheds, New Zealand". Journal of Geophysical Research: Earth Surface 110 (F1). doi:10.1029/2003JF000088. http://onlinelibrary.wiley.com/doi/10.1029/2003JF000088/full. Retrieved 2016-04-03.
- ↑ "Table of nuclides, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. December 5, 2012. Retrieved 2013-01-18.
- ↑ Drever, James (2002). The Geochemistry of Natural Waters. New Jersey: Prentice Hall. pp. 311–322. ISBN 0-13-272790-0.
- 1 2 "Isotope geochemistry, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 26, 2013. Retrieved 2013-08-29.
- 1 2 Avner Vengosh, Allan R Chivas, Malcolm T McCulloch, Abraham Starinsky, Yehoshua Kolodny (September 1991). "Boron isotope geochemistry of Australian salt lakes". Geochimica et Cosmochimica Acta 55 (9): 2591-606. http://www.sciencedirect.com/science/article/pii/001670379190375F. Retrieved 2013-08-29.
- ↑ "Chemical oceanography, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 27, 2013. Retrieved 2013-08-29.
- ↑ Georges Audi, O. Bersillon,J. Blachot, and A.H. Wapstra (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729 (1): 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ↑ W. W. Strong (1909). "On the Possible Radioactivity of Erbium, Potassium and Rubidium". Physical Review (Series I) 29 (2): 170–3. doi:10.1103/PhysRevSeriesI.29.170.
- ↑ David R Lide, H. P. R. Frederikse (1995-06). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. pp. 4–25. ISBN 978-0-8493-0476-7. http://books.google.com/books?id=6khCAQAAIAAJ.
- ↑ "Rubidium, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 23, 2013. Retrieved 2013-08-29.
- ↑ Swadcock (November 16, 2008). "Regional geochemistry, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2013-08-29.
- ↑ Carsten Reinhardt (2008). Chemical Sciences in the 20th Century: Bridging Boundaries. John Wiley & Sons. p. 161. ISBN 3-527-30271-9. http://books.google.co.uk/books/about/Chemical_Sciences_in_the_20th_Century.html?id=gIOK5EUm5ysC&redir_esc=y.
Further reading
- Holland, H.D., & Turekian, K.K. (2004). Treatise on Geochemistry. 9 Volumes. Elsevier
- Marshall, C., & Fairbridge, R. (2006). Encyclopedia of Geochemistry. ISBN 1-4020-4496-8. Berlin: Springer.
- Bernard Gunn: The Geochemistry of Igneous Rocks
- Gunter Faure (1986). Principles of Isotope Geology, John Wiley & Sons. ISBN 0-471-86412-9
- H.R. Rollinson (1993), Using Geochemical Data: evaluation, presentation, interpretation (Longman). ISBN 978-0-582-06701-1
- W.M. White: Geochemistry (Free Download)
External links
- African Journals Online
- Bing Advanced search
- Google Books
- Google scholar Advanced Scholar Search
- JSTOR
- Lycos search
- NASA's National Space Science Data Center
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- Questia - The Online Library of Books and Journals
- SAGE journals online
- The SAO/NASA Astrophysics Data System
- Scirus for scientific information only advanced search
- SIMBAD Astronomical Database
- SpringerLink
- Taylor & Francis Online
- Wiley Online Library Advanced Search
- Yahoo Advanced Web Search
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a chemistry resource . |
| |
Subject classification: this is a Geology resource. |