Fundamentals of chemistry
| |
Subject classification: this is a chemistry resource . |
The Fundamentals of Chemistry is an introduction to the Periodic Table, stoichiometry, chemical states, chemical equilibria, acid & base, oxidation & reduction reactions, chemical kinetics, inogranic nomenclature and chemical bonding.
Periodic Table of Elements

Each chemical element in the universe has unique properties which distinguish it from all of the other chemical elements. Though each is unique, the elements can be still grouped by their commonalities in a useful and meaningful way. The periodic table groups the elements by properties. For the History of the Periodic Table, check out Wikipedia's History of the Periodic Table.
The Periodic Table is available here: Periodic Table on Wikimedia Commons and explanations will be based on this table. A good idea is to have a printed hard copy of the periodic table for easy of access and reference.
Each element has its own box and these boxes make up groups and rows. There are eighteen groups (or families or columns) on the periodic table. Each one represents how many electrons are attached to the elements and correlate to how many valence electrons are present. Electrons are negatively charged subatomic particles that revolve around the nucleus of the element. Valence electrons are electrons that are on the very outside of the atom. There are seven periods (or horizontal rows) that describe electron shells, but more details on electron shells will be discussed in advanced pages.
Traditionally the boxes have certain informative parts about the element. Let's look at hydrogen's box. The "1" in the top corner is the atomic number, which deals with how many protons, or positive charges, are in the atom. The "H" is the symbol for Hydrogen. All the elements get a one or two letter symbol (there are a couple of exceptions with undeclared elements). The number at the bottom is the atomic weight or atomic mass. 1.00794 represents how many grams are in each mole (6.022×1023 entities) of hydrogen. The atomic mass is a very important part of chemistry and has many applications throughout.
There are eight distinct groups that need to be discussed. The first two groups (1A and 2A) as well as the six on the very right (3A-8A). These are called representative elements. Group 1A are alkali metals (except Hydrogen which is a non-metal) and Group 2A are alkaline earth metals. Group 3A through 8A are mixed in properties, but there are specific trends.
Compounds
Chemical equations
Dimensional analysis
Stoichiometry
Stoichiometry is used to analyze quantitative measurements with relation to reactants and products of a chemical equation. The chemical equation is a symbolic representation of a chemical reaction. The reactants of a chemical equation are justified to the left which gives reference to its definition, the substance used or consumed in a chemical reaction. The products of a chemical equation are justified to the right, and is defined as the substance that is yielded or produced in a chemical reaction. In order to completely understand stoichiometric relationships, one must consider the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. Remember that mass or matter is neither created nor destroyed.
Among the properties of elements are states. There are 3 fundamental states of an element: solid, liquid, and a gas. They are indicated by subscript with (s), (l), and (g) respectively and assigned with the appropriate compound or element in the chemical equation. Plasma can also exist, which is an ionized gas with special properties.
Stoichiometry allows chemists to quantitatively analyze relative relationships between substances in a chemical equation.
Balancing chemical equations
Ethyne is added to oxygen gas to yield carbon dioxide and water. This reaction could be written as follows:
- Unbalanced equation
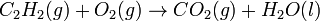
However, the above equation is not balanced.
- On the left side there are two Carbon atoms (C), two Hydrogen atoms (H) and two Oxygen atoms in total.
- On the right there is one Carbon atom, three Oxygen atoms, and two Hydrogen atoms.
Note that in order to properly count up the atoms in an equation, it must be noted to count up atoms with respect to the coefficient and subscripts. Careful notice should be made to compounds and polyatomic ions, since these are grouped together in relation.
In order to balance the equation correctly, a number, known as a coefficient must be added to the front of each representation in a chemical equation.
- Correctly balanced equation
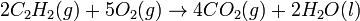
As can be seen, the subscripts were not touched, only whole numbers were added to the front of all the formulas, as needed. The coefficients may be fractions, which are generally used in thermochemistry but for all intents and purposes, whole numbers are generally used.
It would not be correct to balance it by changing the subscript numbers.
- Incorrectly balanced equation
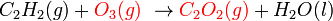
By changing the subscripts you are changing the chemicals involved in the reaction. In the above, 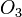 is ozone, not normal oxygen, and
is ozone, not normal oxygen, and 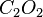 is not a stable compound. A small change in the subscripts and makeup of an individual compound yields a whole different set of properties.
is not a stable compound. A small change in the subscripts and makeup of an individual compound yields a whole different set of properties.
Chemical States
There are five states of matter, plasma being the most common in the known universe. The other three common states are gas, liquid and solid, from least to most dense. A fifth, the Bose-Einstein condensate, can only exist in temperatures approaching absolute zero. It has limited applications in chemistry.
Gases are made up of atoms and/or molecules that are freely moving and therefore have no definite shape. They morph uniformly to the shape of the container that they are in. If the container is not sealed, then the gas can move out. Therefore the volume of the gas is reliant on the temperature and/or pressure throughout the gas or environment. This is observed using the ideal gas laws, which are discussed later.
An important piece of information to know is what an aqueous solution is also. Aqueous solutions are not technically chemical states, but they appear often enough when dealing with stoichiometry and chemistry in general that they should be mentioned.
Acids and Bases
pH
The potential of hydrogen or pH (pronounced /piː.eitʃ/) is a measure of the acidity or alkalinity of a solution, numerically equal to 7 for neutral solutions, increasing pH with rising alkalinity and decreasing pH with more acidity. The pH scale commonly in use ranges from 0 to 14.
An alkali is sometimes called a "base".
| Substance | pH |
|---|---|
| Battery acid | |
| Gastric acid | |
| Lemon juice | |
| Cola | |
| Vinegar | |
| Orange or apple juice | |
| Beer | |
| Acid Rain | |
| Coffee | |
| Tea or healthy skin | |
| Milk | |
| Pure water | |
| Healthy human saliva | |
| Blood | |
| Sea water | |
| Hand soap | |
| Household ammonia | |
| Bleach | |
| Household lye | |
Mathematically, calculate pH using the following equation:
![pH = - log [H^+]](../I/m/1913881e7341bd8c6c4f7a0db9c08bf2.png)
Mathematically, calculate pOH using the following equation:
![pOH = - log [OH^-]](../I/m/d2216626bb52379f503aae63c477e2c1.png)
Combining (adding) the results of pH with pOH should equal fourteen (14).
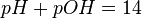
Acids
Characteristics of acids:
- Aqueous acids can turn blue litmus towards red.
- React with bases and certain metals to form salts.
- Arrhenius' definition of acid: Yields hydrogen ions when dissolved in water.
- The Lewis definition of an acid: Can accept a pair of electrons to form a covalent bond.
- Brønsted-Lowry acid definition: A species that can lose or "donate" a hydrogen ion
- Can have a sour taste.
- Can give one or more than one protons (or simply, H+)
- Electrolytes, yet usually are not ionic compounds
Bases
Characteristics of bases:
- Aqueous bases (alkalis) can turn red litmus towards blue.
- React with acids to form salts.
- Arrehenius definition of base: produce OH− ions when dissolved in water.
- Lewis definition of Base: can donate a pair of electrons to form a covalent bond with an acid
- Brønsted-Lowry base definition: A species that can gain or "accept" a hydrogen ion
- Can have a bitter taste.
- Can accept one or more than one protons (or simpler H+)
- Conduct electricity
The difference between bases and alkalis is that alkalis dissolve in water and are considered basic salts of alkaline metals. An example of a base that is not an alkali is ammonia (NH3).
Nomenclature of inorganic chemistry
References
- Flowers, Paul, Klaus Theopold, Richard Langley, William R. Robinson, Mark Blaser, Simon Bott, Donald Carpenetti, Andrew Eklund, Emad El-Giar, Don Frantz, Paul Hooker, George Kaminski, Jennifer Look, Carol Martinez, Troy Milliken, Vicki Moravec, Jason D. Powell, Thomas Sorensen, and Allison Soult. Chemistry. N.p.: n.p., 2015. Chemistry. OpenStax College, Mar. 2015. Web.
See also
- Acids and Bases
- The School of Chemistry