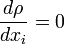Fluid Mechanics for MAP/Introduction
< Fluid Mechanics for MAPDefinition of Fluid
Fluid Mechanics is the study of fluids at rest (fluid statics) and in motion (fluid dynamics).
 Fluid at rest |
 Fluid in motion: Itaipu Dam |
A fluid is defined as a substance that continually deforms (flows) under an applied shear stress regardless of the magnitude of the applied stress. Whereas a solid can resist an applied force by static deformation.
Liquids, gases, plasmas and, to some extent, plastic solids are accepted to be fluids. A perfect fluid offers no internal resistance to change in shape and, consequently, they take on the shape of their containers. Liquids form a free surface (that is, a surface not created by their container) whereas gases and plasmas do not, but, instead, they expand and occupy the entire volume of the container.
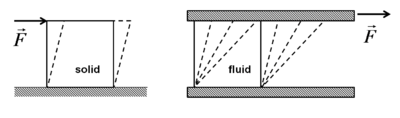 Deformation of a solid and a fluid exposed to an applied force |
 Behavior of liquids, gases and plasmas in a container |
Motivation for studying fluid mechanics
|
The importance of flow phenomena is out of question. Natural phenomena or technological applications are completely or partially involves flow phenomena. It can be met in a diverse range of length of time scales. Atmospheric flows and blood flows are two examples for this diversity. As a tool making specie, humankind learned also how to utilize flow phenomena. Hence, those, who deal with flowing matter, should be better equipped with theoretical understanding and capability to use experimental and numerical investigation tools.  Airbus A380 |
 Hurricane Katrina August 28 2005 NASA |
Historical Background and Future Perspective
|
Fluid mechanics have played an important role in human life. Therefore, it also attracted many curious people. Even in the ancient Greek history, systematic theoretical works have been done. The devlopement of governing equations of fluid flow started already in the 16th century. In the 18th and 19th century, the conservation laws for mass, momentum and energy was already known in its most general form. In the 20th century, developments were in theoretical, experimental and recently numerical. In the theoretical field, mostly solutions of the governing equations for special cases were provided. Experimental methods have been developed to measure flow velocities and fluid properties. By the developement of computers , the numerical treatment of fluid mechanical problems opened new perspectives in research. It is the common blief that in the 21th century, the activities would be most intensive in the development new experimental and numerical tools and application of those for developing new technologies.
|
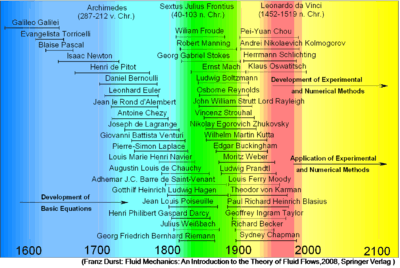 Historical perspective to the developments in the field of fluid mechanics[1] |
Basic components of Fluid Mechanics Research
|
Besides theoretical considerations, exepriments and simulations are heavily used in research. If possible, most productive and accurate approach is the combination of all three methods. However, sometimes environmental conditions can be so harsh for any experimental technique that only theoretical or numerical methods can be used. For example, it is very hard or almost impossible to obtain the velocity or temperature distribution in the die casting mold or in the crucible used for crystal growth, because of very high temperatures. 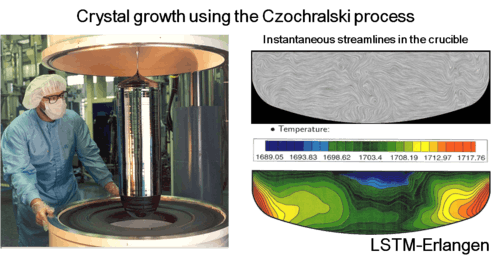 Simulations for diagnosing crystal growth process. |
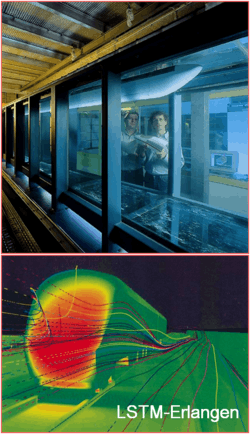 Experimental and numerical investigations conducted on a fast train. |
Viscosity of a Fluid
|
Force applied on a matter creates stresses on it. Stress is simply force per unit area: ![\displaystyle
\tau = \frac{F}{A}\left[\frac{N}{m^{2}}= Pa\right]](../I/m/2dbae7ed06cecf65eaaa4e7b9a302d04.png) Hence the unit of stress is Shear stress is proportional to the deformation rate of the matter, i.e. strain rate: 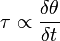 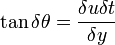
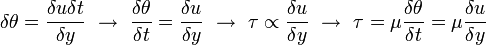
Dynamic viscosity is a thermodynamic property of the material and it depends on temperature and pressure. In general, viscosity of liquids drop by increasing temperature, whereas that of gases increases. The viscosities of liquids and gases increase with increasing pressure. ![\displaystyle \mu=f\left(T,P\right)\left[Pa\cdot s\right]](../I/m/71815c2ab72029eaf223261fbd11013e.png) Often dynamic viscosity is normalized by the density of the fluid and this quantity is called “kinematic viscosity”: ![\displaystyle \upsilon=\frac{\mu}{\rho}\left[\frac{m^{2}}{s}\right]](../I/m/34733d2847053a02cfaf5064e93669e5.png) One can judge the dominance of inertial effects to viscous effects by using a dimensionless number, namely Reynolds number: 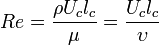
|
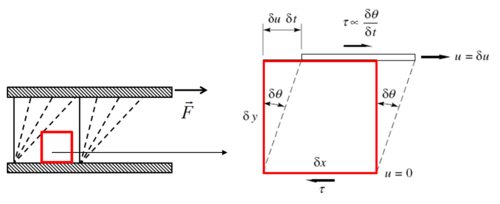 Deformation of a fluid element 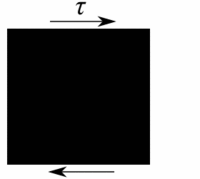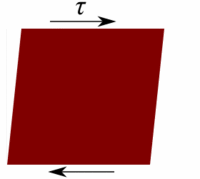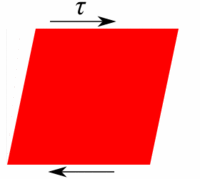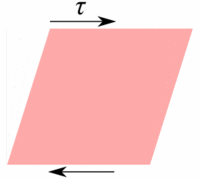 Deformation of a low viscous fluid under the applied stress. 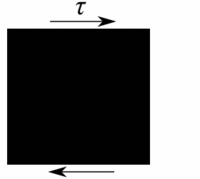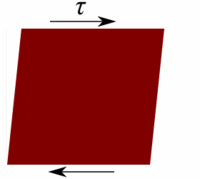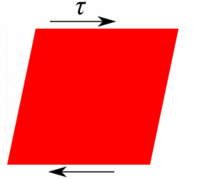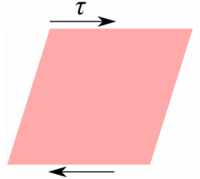 Deformation of a high viscous fluid under the applied stress. |
Elasticity, viscosity, solid- and liquid-like behavior, and plasticity
|
When one tries to deform a piece of material, some of the above properties appear depending on the amplitude and duration of the applied stress.
|
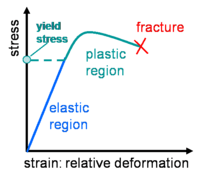 Stress and strain (deformation) relation for a solid substance |
Deborah number
A transition from a more resistant (elastic) to a less resistant behavior (viscous) has a relevant characteristic time scale: the relaxation time of the material. Correspondingly, the ratio of the relaxation time of a material to the timescale of a deformation is called Deborah number :
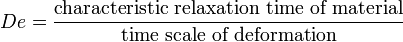
Small Deborah numbers correspond to situations where the material has time to relax (and behaves in a viscous manner), while high Deborah numbers correspond to situations where the material behaves rather elastically. Water can show elastic behavior when the time scale of deformation becomes very short. For example, when one tries to jump to water from a height more than 100 meters, water feels like a solid ground at the instant of collision ( do not try). Corn starch and water mixture (suspension) is a good example with which low and high De number effects can be shown.
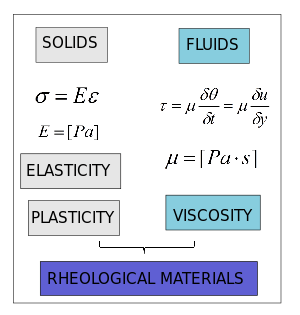
Rheological Material
Fluids can be classified according to the relation between stress 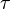 and deformation rate
and deformation rate 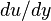 .
The Newtonian fluids show a linear relation
.
The Newtonian fluids show a linear relation
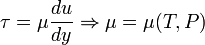
Fluids which do not follow the linear law between stress an the deformation rate are called non-newtonian and they are the subject of rheology. A dilatant (shear-thickening) fluid increases resistance with increasing applied stress. Alternately, a pseudoplastic (shear-thinning) fluid decreases resistance with increasing stress. If the thinning effect is very strong, the fluid is termed plastic. The limiting case of a plastic substance is one which requires a finite yield stress before it begins to flow. The linear-flow Bingham plastic idealization is shown in the figure, but the flow behavior after yield may also be nonlinear. Examples of a yielding fluid are toothpaste and ketchup, which will not flow out of the tube until a finite stress is applied by squeezing.
Some fluids show decreasing (thixotropic) or increasing resistance (rheopectic) in time for the same deformation rate. For example, pudding is a rheopectic fluid and some paints are thixotropic.
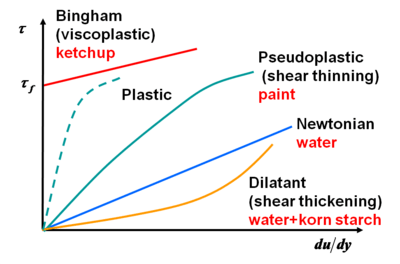 Types of different fluids regarding their stress-strain dependence |
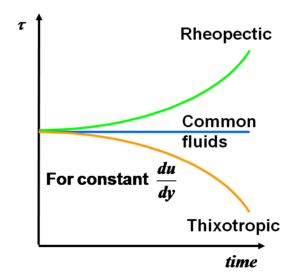 Types of different fluids regarding the change of the stress in time for constant a strain |
Continuum Assumption
|
In many technical applications, the distance between the molecules (mean free path) ( The molecules are not fixed in a lattice but move about freely relative to each other. Thus fluid density, or mass per
unit volume, has no precise meaning because the number of molecules occupying a given volume continually changes.
If the selected unit volume |
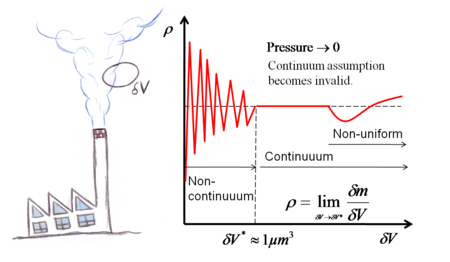 Continuum assumption in a fluid flow |
Pressure in a fluid
Pressure is force per unit area and is a scalar quantity.
![\displaystyle
p=\frac{F}{A}\left[Pa\right]\ ; \left(1 bar = 10^{5} Pa\right)](../I/m/4370c9e56db4329c857435019453bb0a.png)
In a fluid at rest, the tangential viscous forces are absent and the only force between adjacent surfaces is normal to the surface. In a resting fluid there is only a normal stress (pressure). In other words, force caused by the pressure on a surface is normal to that surface.
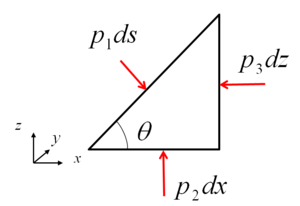
Balance in x-direction:
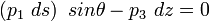
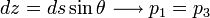
Balance in z-direction:
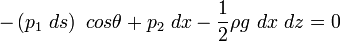
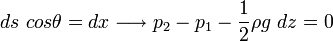
For an infinitesimal prism, effect of gravity can be neglected.
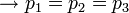
Interface phenomena and surface tension
|
Surface tension phenomena occur at the interface of one liquid and another liquid, gas or a solid wall. The cohesive forces between molecules down into a liquid are shared with all neighboring atoms. Those on the surface have no neighboring atoms above, and exhibit stronger attractive forces upon their nearest neighbors on the surface. This enhancement of the intermolecular attractive forces at the surface is called surface tension.  Examples of interface and surface tension phenomena |
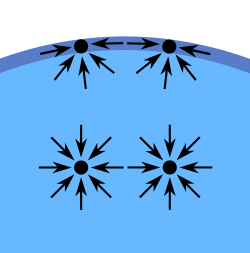 Inter molecular attraction in the liquid and on the liquid surface |
If the interface is curved, a mechanical balance shows that there is a pressure difference across the interface, the pressure being higher on the concave side,
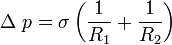
where ![\displaystyle \sigma\left[\frac{N}{m}\right]](../I/m/394eb4b55e21ea3e9864519221dcfb08.png) is the surface tension coefficient. Surface tension coefficient is not a property of the liquid alone, but a property of the liquid's interface with another medium.
is the surface tension coefficient. Surface tension coefficient is not a property of the liquid alone, but a property of the liquid's interface with another medium.
According to the above equation, in the soap bubble or in the droplet, inner pressure is higher than outer pressure. This can also be shown by a force balance. In the droplet, the force balance in the vertical direction reads
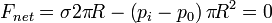
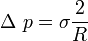
Similarly, in the soap bubble the force balance becomes
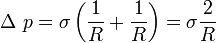
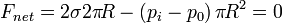
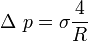
Note that owing to the two interfaces in the soap bubble force due to surface tension is as double as that in the droplet.
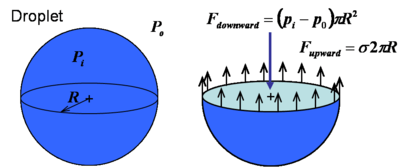 Force balance in the droplet |
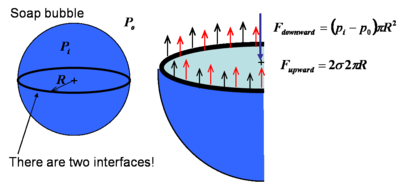 Force balance in the bubble |
The contact angle is the angle between the liquid-solid and gas-liquid interfaces. It is calculated such that angle remains in the liquid. It is dependent on the adhesion forces between the liquid molecules and the solid wall. These forces are sensitive to the actual physicochemical conditions of the solid-liquid interface.
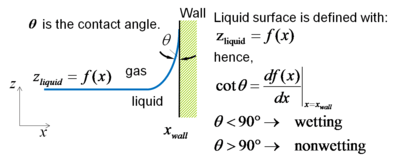 Definition of contact angle |
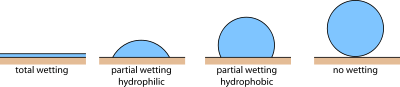 Wetting |
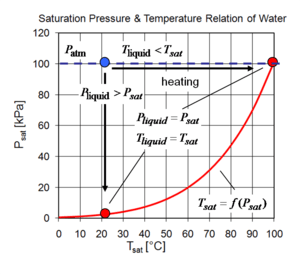
Saturation pressure and cavitation
|
Evaporation occurs at the liquid gas interface. When the vapor pressure of liquid is less than the liquid's saturation pressure at the given liquid temperature, the evaporation and condensation occurs at the same time on the interface.At the liquid solid interface, at a given temperature, liquids starts to boil at saturation pressure.
Instead of increasing the temperature of the liquid, one can decrease the pressure of the liquid so that it starts to boil, or so to say cavitates.
|
Cavitation Propeller Damage |
Streamline, streakline, pathline and timeline
|
Four basic types of line patterns are used to visualize flows:
In a steady flow streamlines, streaklines and pathlines are identical. |
Flow visualization around a car done by smoke. The lines are streaklines. |
Laminar and turbulent flows
|
Laminar flows are:
In turbulent flows:
Laminar to turbulent transition occurs when the disturbances in the flow can not be damped anymore by viscous forces. This happens when the inertia of the flow is increased and/or the flow configuration (boundaries, states of the fluid(s)) causes the generation and/or amplification of very small disturbances. As Reynolds number (Re) is the ratio of the inertial forces to viscous forces, for different types of flows, over a critical Reynolds number, transition to turbulence takes place. Below a list of simple but still technically interesting flow cases and critical Reynolds numbers are listed:
where |
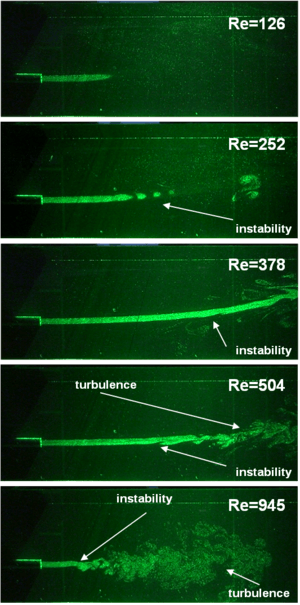 Laminar to turbulent transition of a submerged jet flow. |
Compressibility
Ideal Gas law (Equation of State)
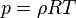 : Where R is the gas constant and T is the universal temperature.
: Where R is the gas constant and T is the universal temperature.
![R_{air} = 286,9 \left[\frac{J}{kg}K\right]](../I/m/335807c0aac8ea8b6dbbde14319a3826.png)
|
Density and volume change:
Liquids can be accepted to be incompressible in many applications. Air can be compressible, especially when there are large changes of pressure in the flow.
|
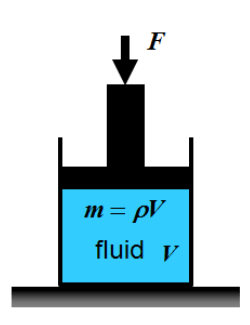 Figure: Gas under pressure |
Classification of flows
|
Following chart covers most of the flow phenomena, which might occur in a flow problem. When one deals with a flow problem, first task is to classify the flow. Correct classification helps to choose the correct and most efficient methods to deal with this problem. 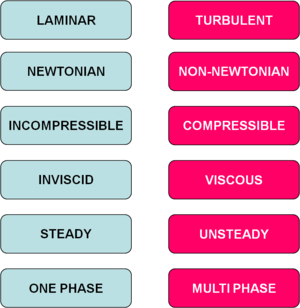 References
|


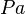 . There can be normal and shear stresses in and on the matter.
. There can be normal and shear stresses in and on the matter.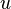 is the deformation speed. For very small deformation angles
is the deformation speed. For very small deformation angles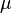 is the dynamic viscosity of the fluid.
is the dynamic viscosity of the fluid. 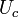 and
and 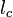 are characteristic velocity and length scales of the flow.
are characteristic velocity and length scales of the flow.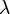 ) are much larger than the molecular diameter. For air,
) are much larger than the molecular diameter. For air, 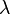 is around
is around 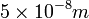 .
.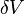 is smaller than the cube of the mean free path between the molecules, there will be large scatter in the determination of density, since the molecules move freely relative to each other, i.e. at one instant the number of molecules in the unit volume is not constant. This effect becomes unimportant if the unit volume is large compared with, say, the cube of the molecular spacing, when the number of
molecules within the volume will remain nearly constant in spite of the enormous interchange
of particles across the boundaries. In other words, when
is smaller than the cube of the mean free path between the molecules, there will be large scatter in the determination of density, since the molecules move freely relative to each other, i.e. at one instant the number of molecules in the unit volume is not constant. This effect becomes unimportant if the unit volume is large compared with, say, the cube of the molecular spacing, when the number of
molecules within the volume will remain nearly constant in spite of the enormous interchange
of particles across the boundaries. In other words, when  . Over this value, the medium can be accepted as continuum , such that the variations in space and time can be accepted to be smooth and differential equations can be written to describe the fluid motion. If, however, the chosen unit volume is too large, there could be a noticeable variation within the selected volume owing to the non-uniform bulk distribution of molecules caused by temperature and/or pressure variations in the flow field.
. Over this value, the medium can be accepted as continuum , such that the variations in space and time can be accepted to be smooth and differential equations can be written to describe the fluid motion. If, however, the chosen unit volume is too large, there could be a noticeable variation within the selected volume owing to the non-uniform bulk distribution of molecules caused by temperature and/or pressure variations in the flow field.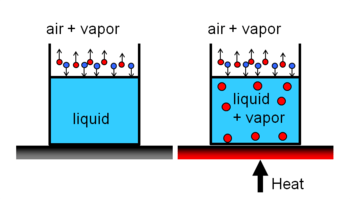
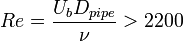
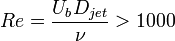
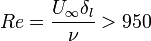
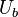 ,
, 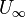 are the bulk velocity of the fluid or the velocity of fluid approaching to the plate.
are the bulk velocity of the fluid or the velocity of fluid approaching to the plate. 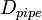 ,
, 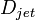 and
and 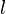 are the pipe diameter, jet diameter or the length of the plate.
are the pipe diameter, jet diameter or the length of the plate. 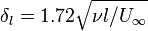 is the displacement thickness.
is the displacement thickness.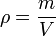
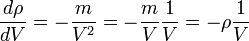
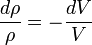
![\displaystyle E_{v} = - \frac{dp}{\frac{dV}{V}} = \frac{dp}{\frac{d\rho}{\rho}} \ [Pa]](../I/m/4e9a20f5782ed295886a85cdc61ae7a6.png)
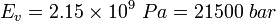
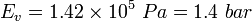
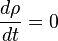 and
and