Electromagnetic radiation
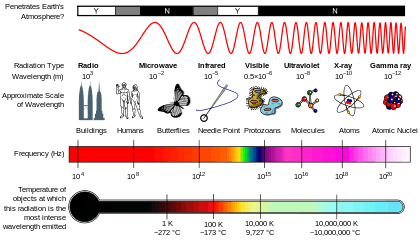
Electromagnetic radiation comes in many different types, although the differences between them are quantitative rather than qualitative. This teaching aid lists the different types that are generally recognised.
Electromagnetic radiation is a wave, so has a wavelength λ and a frequency ν. It is also composed of particles called photons, and each photon has an energy E. These three quantities are related, and any of them may be used to define the type of radiation.
Units of measurenent
Scientists usually use SI units. The unit of frequency is the hertz (Hz), one cycle per second, named after Heinrich Hertz. For our purposes, we shall use much higher frequencies. One thousand Hz is a kHz (kilohertz); one thousand kHz is a MHz (megahertz); one thousand MHz is a GHz (gigahertz); one thousand GHz is a THz (terahertz).
The SI unit of length is the metre (m). One thousand metres is a kilometre (km). One hundredth of a metre is a centimetre (cm); one thousandth is a millimetre (mm), one millionth is a micrometre (μm, often called a micron and written μ). A thousandth of a micrometre is called a nanometre (nm). A tenth of a nm is known as an Angstrom unit or Angstrom (Å). The use of cm and Angstrom is discouraged in the SI system, but many scientists like them.
Exercise: Find out why the cm and Angstrom are discouraged in the SI system.
The SI unit of energy is the Joule (J). However, there is a much smaller unit, the electron volt (eV). Again, its use is discouraged in the SI system, but many scientists like it. The eV is the energy gained by an electron in passing through a potential difference of one volt. Since the charge on an electron is 1.60218 x 10-19 Coulombs, an eV is 1.60218 x 10-19 J. A keV is 1000 eV and a MeV is 1000 keV.
As will be discussed below, either frequency or wavelength may be used for radio waves. Wavelength is usually used for infrared, visible and ultraviolet (expressed in nm or Å), and photon energy for X-rays and gamma rays (expressed in keV or MeV).
Exercise: Find out why the eV is discouraged in the SI system. What would be an appropriate SI unit to replace the keV or MeV? Why are these units more convenient for physicists than the SI ones?
Relationships
By definition, wavelength x frequency = speed. Since the speed of light c = 2.997458 x 108 m/s, we have
- λ ν = c
Also, by Planck's law,
- E = h ν
where h is Planck's constant 6.62607 x 10-34 Js.
It follows that a photon with an energy of 1eV has a frequency of 1 eV/h = 2.41799 x 1014 Hz or about 242 THz and a wavelength of c.h/1 eV = 1.23984 x 10-6 m or about 1,240nm or 12,400Å. As will be seen below, that would put the photon in the infrared range. In practice, photon energies are never quoted for such long wavelengths.
Exercise: Find why photon energies are only important at very short wavelengths.
Bands in the spectrum
The electromagnetic spectrum is divided into several bands. These definitions are generally informal and vague, and the bands may overlap. There seems to be a gap between the ultra-violet and X-ray bands. Sometimes, the choice of band depends on how the radiation is generated. For example, radiation from radioactive decay is always referred to as gamma rays, even if its photon energy would class it as X-rays.
The main bands used, in order of decreasing wavelength (hence increasing frequency and photon energy) are:
- Radio
- Microwaves
- Infra-Red
- Visible light
- Ultra-violet
- X-rays
- Gamma rays
A convenient mnemonic for remembering these is "Rabbits Mate In Very Unusual eXpensive Gardens".
There are many bands within these, which are even vaguer and more prone to overlap. Each band is discussed in more detail below. The limits given are rough, so exact consistency between wavelength, frequency and photon energy cannot be expected.
Radio
The existence of radio waves was predicted by James Clerk Maxwell in 1864. Their properties were investigated by Heinrich Hertz (after whom the hertz is named) in 1885-9.
With the splitting-off of microwaves as a separate band, radio waves are now regarded as having wavelengths exceeding 30cm, frequencies below 1GHz. There is no upper limit to the wavelength hence no lower limit to the frequency. The standard divisions are:
- Ultra high frequency (UHF): Frequencies of 300MHz to 3GHz, hence wavelengths of 10cm to 1m. This overlaps with the microwave region.
- Very high frequency (VHF): Frequencies of 30MHz to 300MHz, hence wavelengths of 1m to 10m.
- High frequency (HF): Frequencies of 3MHz to 30MHz, hence wavelengths of 10m to 100km.
- Medium frequency (MF): Frequencies of 300kHz to 3MHz, hence wavelengths of 100m to 1km. These are also called hectometric waves (hectometre = 100m).
- Low frequency (LF): Frequencies of 30kHz to 300kHz, hence wavelengths of 1km to 10km.
- Very low frequency (VLF): Frequencies of 10kHz to 30kHz, hence wavelengths of 10km to 30km.
- Ultra low frequency (ULF): Frequencies of 300Hz to 10kHz, hence wavelengths of 30km to 1000km; these are mainly used for long-distance underwater transmission.
- Extremely low frequency (ELF): The lowest frequencies (below 300 Hz; wavelength > 1000km).
Sometimes a division by wavelength is used:
- Short wave (SW): Wavelengths of 10-200m hence frequencies of 1.5-33MHz; there are several sub-bands.
- Medium wave (MW): Wavelengths of 200-1000m hence frequencies of 300kHz-1.5MHz.
- Long wave (LW): Wavelengths > 1000m hence frequencies < 300kHz.
Other terms found are:
- Frequency modulation (FM): This is not a frequency band, but a way of coding the audio signal onto a radio wave. However, quite a high frequency is necessary for FM to work well, and in practice frequencies of close to 100MHz (wavelength 3m, the middle of the VHF band) are used.
- Digital radio: Again, this is not a frequency band. Typically, frequencies of the order of 200MHz (wavelength 1.5m, towards the short wavelength or high frequency end of the VHF band) are used.
- Decametric: Wavelengths of 10-30m (decametre = 10m) hence frequencies of 10-33MHz. The planet Jupiter radiates strongly at these wavelengths.
Microwaves
Microwaves in radio telecommunications are the very shortest wavelengths usable for communication. They have wavelength 1mm to 30cm, or frequency 1-300 GHz.
An important frequency is 1420 MHz (wavelength 21.1cm). Interstellar hydrogen radiates at this frequency, making it a very useful frequency for radio astronomers.
Microwaves have only fairly recently been regarded as distinct from very short wavelength radio waves. Their recognition is probably due to the popularity of microwave ovens. In fact, these ovens typically use frequencies of 300MHz to 1GHz, or wavelengths 30cm to 100cm, frequencies in the lower half of the UHF band hence slightly below the radio telecommunications definition.
Infra-red
Infra-red radiation, the first non-visible electromagnetic radiation to be discovered, was discovered by William Herschel in 1800.
It has wavelengths below 1mm, down to where they start to become visible (about 700nm). Thus the frequency range is from 300 GHz up to about 430 THz. Shorter wavelengths are near infra-red; middle ones are mid infra-red; longer ones are far infra-red.
The longest wavelengths (0.1-1mm, 300GHz-3THz) are sometimes regarded as a distinct band called Submillimetre radiation (from their wavelength) or Terahertz radiation (from their frequency). They can penetrate through some objects opaque to visible light, but can be used to produce images, so are a safe alternative to some uses of X-rays.
Visible light
This is of course the most familiar form of electromagnetic radiation. It covers wavelengths of around 400-700nm (frequencies of 430-750 THz); different people have different limits. The range of wavelengths that a person can detect has is nothing to do with colour blindness, which refers to the ability to perceive differences in frequency as colour rather than ability to detect the radiation.
The longest wavelengths correspond to red, then orange, yellow, green, blue, indigo and violet are shorter and shorter. There is no sharp division into different colours; they merge gradually into each other as the wavelength increases.
This is a relatively narrow band, covering a factor of no more than two in wavelength or frequency. However, it is comparatively well defined.
It has been reported that some people who have had cataracts removed can see shorter wavelengths than other people, because they get replacement lenses in their eyes that are more transparent to these wavelengths than natural lenses. A well-documented case is the astronomer Walter Scott Houston.
Ultraviolet (UV)
Ultraviolet radiation was discovered by Johann Wilhelm Ritter in 1801.
There are several terms used.
- Near ultraviolet: 400-300nm
- Middle ultraviolet: 300-200nm
- Extreme or far ultraviolet: 200-100nm
- Vacuum ultraviolet is UV with wavelengths shorter than about 150-200nm. Such wavelengths are strongly absorbed by air, especially oxygen, so are best used in a vacuum. However, it is possible to use pure nitrogen rather than a vacuum.
UVA, UVB, UVC are terms used to assess how dangerous the UV is to human skin.
- UVA: 320-400 nm: Not absorbed by ozone layer; fairly harmless
- UVB: 280-320 nm: Partly absorbed by ozone layer; more dangerous
- UVC: 100-280 nm: Totally absorbed by ozone layer; most dangerous
Short-wave UV, X-ray and gamma-ray radiation are sometimes lumped together as ionising radiation, because the photons are energetic enough to knock electrons out of atoms, ionising them. Such radiation is dangerous to living things, because the ionisation can disrupt biochemical processes and even cause cancer. Alpha and beta particles can also be called ionising radiation.
X-rays
These have a wavelength of 0.01 to 10 nm, hence photon energies of 1keV to 1000keV. They were discovered by Wilhelm Roentgen in 1895.
Often, X-rays are divided into hard X-rays (wavelength of 0.01 to 0.1 nm, photon energies of 10keV to 1000keV) and soft X-rays (wavelength of 0.1 to 10 nm, photon energies of 0.1keV to 10keV). In general, shorter wavelength X-rays are said to be harder than longer wavelength ones.
X-rays are widely used in medicine and technology because they can penetrate many materials hence reveal internal structures. Excessive exposure of living beings to X-rays, especially very hard ones, can be damaging and even fatal. Long-term exposure to even moderate levels can cause cancer in humans.
Gamma rays
These have a wavelength of 0.01 nm or less, hence photon energies exceed 100keV. They were discovered by Henri Becquerel in 1898 as a form of radioactivity. (He discovered three types, which he called alpha, beta and gamma, hence the name. However, alpha and beta are particles, not electromagnetic rays.)
Gamma rays are even more dangerous to living things than are X-rays, and are sometimes used to sterilise equipment.
There is no lower boundary to the wavelength hence no upper bound to frequency or photon energy. In October 2011, photons with energies exceeding 100 billion eV, i.e. over a million times the lower limit, were detected from the Crab pulsar.