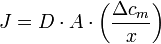Diffusion and osmosis
Diffusion
| | Educational level: this is a tertiary (university) resource. |
| | Subject classification: this is a science resource . |
| | Resource type: this resource is a lesson. |
Simple diffusion is know as the random movement of solute molecules in a solvent tending towards an equilibrium (evenly-distributed) state, a better way to understand this is that diffusion is the net movement of a solute from a region of greater concentration to a region of lower concentration. The rate of Diffusion (J) is proportional to the conetration gradient over distance x (membrane thickness)
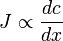
Rate of diffusion is independent of diffusion of other substances although is related to
- Physical properties of solute and solvent molecules (eg size, electric charge)
- temperature
- electric field
Diffusion of a solute is rapid over short distances, although the membrane is a selective barrier to diffusion and the concetration gradient lies within the membrane barrier. To truly understand the mechanism of diffusion it must be described quantitavely as Fick's Law of Diffusion
Fick's Law of Diffusion
From a biological perspective Fick's First law 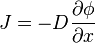 gave rise to the Formula
gave rise to the Formula 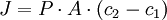
- J = the rate of diffusion (mol/sec)
- P = the permeability coefficient of solute across membrane (m/sec)
- A = membrane area (m2)
- C2-C1 = The concentration difference often termed
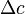
J is also related to the diffusion coefficient in the membrane (D),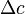 within the membrane and the membrane thickness (x) and can be calcualted by means of this equation
within the membrane and the membrane thickness (x) and can be calcualted by means of this equation
The Diffusion coefficient has various factors contributing to it and can be calcualted using the formula
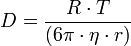
- R = Gas constant (8.3 J/K.mol)
- T = Absolute temperature (K)
- η = Viscosity of barrier
- r = Radius of diffusing molecule
The hydrophobic lattice structure of the bilayer acts like a viscous molecular "seive" , also values for P and D are specific for membrane AND diffusing molecules. The solute concentration within a membrane depends on Kp. Kp is the lipid-water coefficient and is described as
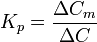 .
.
For a hydrophobic molecule Kp < 1 and for a hydroophilic molecule Kp < 1, permeability can then be calculated including Kp in the equation and thus gives us 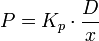
Important factors for predicting passive permeability
frictional effects
- Molecular size - Small,P↑ ; large . P↓
- Molecular shapre - Straight, P↑ ; globular P↓
- Membrane Viscostiy - Short R chains, -C=C-, Tο
Lipid solubility (Kp)
- Kp high such as O2, CO2, anaesthetics, lipophilic group then P↑
- Kp low such as sugars, amino acids, ions , polar/charged groups then P↓
==Osmosis==yeahayeha Osmosis is the net solvent flow, water moves from regions of higher to lower (more negative) water potenials, showing bulk flow. In animals water potential = Osmostive potential (zero for pure water; incresingly negative as solute concentration increases)
Osmolarity is proportional to the concentration of dissolved solutes and inversely proportional to osmotic potential.A common example to describe osmolarity is red blood cells in different salt concetrations if a solution contains a higher concentration of salt than the cells ICF water flows out of the cells and the cell shrinks, this solution is known as Hyperosmotic. Solutions that are at equilibrium with the cell salt concentration are known Isosmotic. If cells are placed in distil water the salt concetration will be larger inside the cell and water will flow into the cell causing it to burst this type of solution is know as Hypoosmotic.
Diffusion and Osmosis Quiz