Chemicals/Thoriums
< Chemicals
Thorium is chemical element number 90 in the periodic table.
Radiation
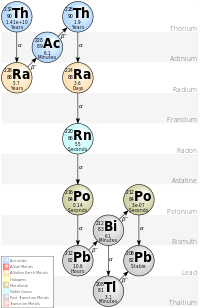
The decay chain on the right depicts that chain for thorium.
Theoretical thorium
Def. "a chemical element (symbol Th) with atomic number 90"[1] is called thorium.
Metals
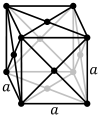
Thorium is a silvery, radioactive, metallic element. At room temperature and pressure, thorium crystallizes into a face-centered cubic lattice, where one thorium atom occupies each location of a black sphere in the diagram on the left.
Isotopes
| nuclide symbol |
historic name |
Z(p) | N(n) | isotopic mass (u) |
half-life[n 1] | decay mode(s)[2][n 2] |
daughter isotope(s)[n 3] |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|---|
| excitation energy | ||||||||||
| 209Th | 90 | 119 | 209.01772(11) | 7(5) ms [3.8(+69-15)] |
5/2-# | |||||
| 210Th | 90 | 120 | 210.015075(27) | 17(11) ms [9(+17-4) ms] |
α | 206Ra | 0+ | |||
| β+ (rare) | 210Ac | |||||||||
| 211Th | 90 | 121 | 211.01493(8) | 48(20) ms [0.04(+3-1) s] |
α | 207Ra | 5/2-# | |||
| β+ (rare) | 211Ac | |||||||||
| 212Th | 90 | 122 | 212.01298(2) | 36(15) ms [30(+20-10) ms] |
α (99.7%) | 208Ra | 0+ | |||
| β+ (.3%) | 212Ac | |||||||||
| 213Th | 90 | 123 | 213.01301(8) | 140(25) ms | α | 209Ra | 5/2-# | |||
| β+ (rare) | 213Ac | |||||||||
| 214Th | 90 | 124 | 214.011500(18) | 100(25) ms | α | 210Ra | 0+ | |||
| 215Th | 90 | 125 | 215.011730(29) | 1.2(2) s | α | 211Ra | (1/2-) | |||
| 216Th | 90 | 126 | 216.011062(14) | 26.8(3) ms | α (99.99%) | 212Ra | 0+ | |||
| β+ (.006%) | 216Ac | |||||||||
| 216m1Th | 2042(13) keV | 137(4) µs | (8+) | |||||||
| 216m2Th | 2637(20) keV | 615(55) ns | (11-) | |||||||
| 217Th | 90 | 127 | 217.013114(22) | 240(5) µs | α | 213Ra | (9/2+) | |||
| 218Th | 90 | 128 | 218.013284(14) | 109(13) ns | α | 214Ra | 0+ | |||
| 219Th | 90 | 129 | 219.01554(5) | 1.05(3) µs | α | 215Ra | 9/2+# | |||
| β+ (10−7%) | 219Ac | |||||||||
| 220Th | 90 | 130 | 220.015748(24) | 9.7(6) µs | α | 216Ra | 0+ | |||
| EC (2×10−7%) | 220Ac | |||||||||
| 221Th | 90 | 131 | 221.018184(10) | 1.73(3) ms | α | 217Ra | (7/2+) | |||
| 222Th | 90 | 132 | 222.018468(13) | 2.237(13) ms | α | 218Ra | 0+ | |||
| EC (1.3×10−8%) | 222Ac | |||||||||
| 223Th | 90 | 133 | 223.020811(10) | 0.60(2) s | α | 219Ra | (5/2)+ | |||
| 224Th | 90 | 134 | 224.021467(12) | 1.05(2) s | α | 220Ra | 0+ | |||
| β+β+ (rare) | 224Ra | |||||||||
| 225Th | 90 | 135 | 225.023951(5) | 8.72(4) min | α (90%) | 221Ra | (3/2)+ | |||
| EC (10%) | 225Ac | |||||||||
| 226Th | 90 | 136 | 226.024903(5) | 30.57(10) min | α | 222Ra | 0+ | |||
| 227Th | Radioactinium | 90 | 137 | 227.0277041(27) | 18.68(9) d | α | 223Ra | 1/2+ | Trace[n 4] | |
| 228Th | Radiothorium | 90 | 138 | 228.0287411(24) | 1.9116(16) a | α | 224Ra | 0+ | Trace[n 5] | |
| CD (1.3×10−11%) | 208Pb 20O | |||||||||
| 229Th | 90 | 139 | 229.031762(3) | 7.34(16)×103 a | α | 225Ra | 5/2+ | |||
| 229mTh | 0.0076(5) keV | 70(50) h | IT | 229Th | 3/2+ | |||||
| 230Th[n 6] | Ionium | 90 | 140 | 230.0331338(19) | 7.538(30)×104 a | α | 226Ra | 0+ | Trace[n 7] | |
| CD (5.6×10−11%) | 206Hg 24Ne | |||||||||
| SF (5×10−11%) | (Various) | |||||||||
| 231Th | Uranium Y | 90 | 141 | 231.0363043(19) | 25.52(1) h | β− | 231Pa | 5/2+ | Trace[n 4] | |
| α (10−8%) | 227Ra | |||||||||
| 232Th[n 8] | Thorium | 90 | 142 | 232.0380553(21) | 1.405(6)×1010 a | α | 228Ra | 0+ | 1.0000 | |
| β−β− (rare) | 232U | |||||||||
| SF (1.1×10−9%) | (various) | |||||||||
| CD (2.78×10−10%) | 182Yb 26Ne 24Ne | |||||||||
| 233Th | 90 | 143 | 233.0415818(21) | 21.83(4) min | β− | 233Pa | 1/2+ | |||
| 234Th | Uranium X1 | 90 | 144 | 234.043601(4) | 24.10(3) d | β− | 234mPa | 0+ | Trace[n 7] | |
| 235Th | 90 | 145 | 235.04751(5) | 7.2(1) min | β− | 235Pa | (1/2+)# | |||
| 236Th | 90 | 146 | 236.04987(21)# | 37.5(2) min | β− | 236Pa | 0+ | |||
| 237Th | 90 | 147 | 237.05389(39)# | 4.8(5) min | β− | 237Pa | 5/2+# | |||
| 238Th | 90 | 148 | 238.0565(3)# | 9.4(20) min | β− | 238Pa | 0+ | |||
- ↑ Bold for nuclides with half-lives longer than the age of the universe (nearly stable)
- ↑ Abbreviations:
CD: Cluster decay
EC: Electron capture
IT: Isomeric transition
SF: Spontaneous fission - ↑ Bold for stable isotopes
- 1 2 Intermediate decay product of 235U
- ↑ Intermediate decay product of 232Th
- ↑ Used in Uranium-thorium dating
- 1 2 Intermediate decay product of 238U
- ↑ Primordial radionuclide
Research
Hypothesis:
- Thorium can be fissioned and fusioned.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[3] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[4]"[5]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[6] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[7]
See also
References
- ↑ Emperorbma (9 July 2015). "thorium, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-07-16.
- ↑ http://www.nucleonica.net/unc.aspx
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
| |||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a chemistry resource . |