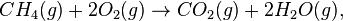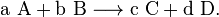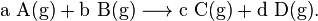Chemicals/Reactions
< Chemicals
Chemical reactions are associated with some changes in the chemical nature/properties of substances. For example, under specific circumstances, when hydrogen chemically reacts with oxygen, water is formed. The chemical propeties of water are quite different from its constituent elements, i.e. hydrogen and oxygen.
Chemical reactions can be classified into two main types: endothermic and exothermic reactions. An endothermic reaction is sustained only when the required quantity of heat is continuously supplied to the reaction zone and the reation absorbs heat. Exothermic reactions release heat and require removal of heat from the reaction zone for sustenance.
Equations
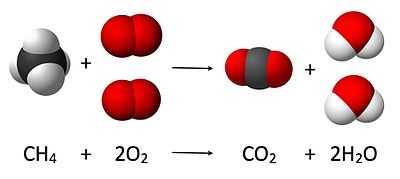
Centered above is a visual that shows how the law of conservation of atoms works: there must be the same number of atoms of each element for the reactants and the products.
Each term in the reaction equation has two parts: a coefficient indicating the number of molecules and a chemical formula for each molecule involved in the reaction.
A molecule of methane (CH4) is combined with two molecules of oxygen (O2) to produce a molecule of carbon dioxide (CO2) and two molecules of water vapor (H2O).
The physical state of the chemicals has not been included. Depending upon the conditions for which the equation records the reaction, all molecules could be gases. This may be represented as
where (g) stands for gas.
Written more generally,
Here, a = 1, A = CH4, b = 2 , B = O2, c = 1, C = CO2, d = 2, and D = H2O. Including the gaseous state of each molecule yields
The liquid methane/liquid oxygen reaction motor or engine pictured at the page top is probably preheating each right before the components enter the reaction chamber which is just above the exhaust manifold.
Elementary reactions
Def. a "reaction for which no reaction intermediates have been detected or need to be postulated in order to describe the chemical reaction on a molecular scale"[1] is called an elementary reaction.
"An elementary reaction is assumed to occur in a single step and to pass through a single transition state."[1]
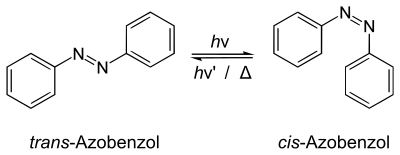
The reaction at the top of this section shows trans-azobenzene being transformed into cis-azobenzene induced by light (hν) or heat (Δ). Trans means widely separated and cis means close by. The double arrow (⇌) indicates that the reaction is reversible.
Phosphate reactions
A phosphate reaction is a chemical reaction that leads to the transformation of one set of phosphates to another.
Research
Hypothesis:
- Chemical reactions often occur in small steps.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[2] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[3]"[4]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[5] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[6]
See also
References
- 1 2 M. Nic, J. Jirat, B. Kosata, and A. Jenkins (24 February 2014). [http://goldbook.iupac.org/E02035.html isbn=0-9678550-9-8 "IUPAC Gold Book"]. IUPAC. doi:10.1351/goldbook. Retrieved 2015-08-08.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
| |||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a chemistry resource . |