Chemicals/Nitrogens
< Chemicals
On the right liquid nitrogen is shown being poured.
Nitrogen is element number seven based on the number of protons in its nucleus.
Nitrogens is a lecture on the general nature and specific characteristics of various natural and hominin-made nitrogens. It is an offering from the school of chemistry.
Emissions

A nitrogen green emission line occurs in plasmas at 566.934 nm from N VIII.[1]
"[T]he 5198-, 5201-A lines of nitrogen [occur] in the [Earth's] nightglow."[2]
Nitrogen has an emission line at 658.4 nm.
Nitrogen has two emission lines that occur in plasmas at 455.368 and 455.545 nm from N VII.[1]
There is an "(0,2) vibrational component of the B-x electronic transition of N2(+) at 470.9 nm."[3]
Nitrogen has an emission line that occurs in plasmas at 388.678 nm from N VII.[1]
As seen in its spectrum above, nitrogen has many emission lines in the violet.
Gases
"Molecular nitrogen (N2) [is] a colorless, odorless gas at room temperature."[4]
Liquids
At the top of this page, liquid diatomic nitrogen (N2) is being poured.
Nebulas
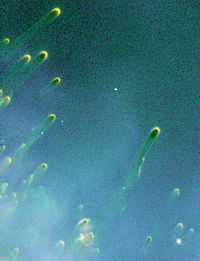
At right is an image gaseous objects ("cometary knots") discovered in the thousands. These knots are imaged with the Hubble Space Telescope while exploring the Helix nebula, the closest planetary nebula to Earth at 450 light-years away in the constellation Aquarius. Although ground-based telescopes have revealed such objects, astronomers have never seen so many of them. The most visible knots all lie along the inner edge of the doomed star's ring, trillions of miles away from the star's nucleus. Although these gaseous knots appear small, they're actually huge. Each gaseous head is at least twice the size of our solar system; each tail stretches for 100 billion miles, about 1,000 times the distance between the Earth and the Sun. The image was taken in August 1994 with Hubble's Wide Field Planetary Camera 2. The red light depicts nitrogen emission ([NII] 658.4 nm).
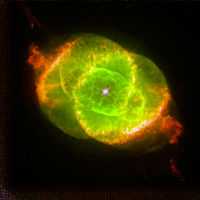
The second image at right is a color picture, taken with the Wide Field Planetary Camera-2. It is a composite of three images taken at different wavelengths. (red, hydrogen-alpha; blue, neutral oxygen, 630.0 nm; green, ionized nitrogen, 658.4 nm). This NASA Hubble Space Telescope image shows one of the most complex planetary nebulae ever seen, NGC 6543, nicknamed the "Cat's Eye Nebula." The image was taken on September 18, 1994. NGC 6543 is 3,000 light-years away in the northern constellation Draco. The term planetary nebula is a misnomer; dying stars create these cocoons when they lose outer layers of gas.
Research
Hypothesis:
- To form a nitrogen plasma, diatomic molecular nitrogen gas must be dissociated into monatomic nitrogen gas, then one or more electrons must be either added or taken away.
Control groups

The findings demonstrate a statistically systematic change from the status quo or the control group.
“In the design of experiments, treatments [or special properties or characteristics] are applied to [or observed in] experimental units in the treatment group(s).[5] In comparative experiments, members of the complementary group, the control group, receive either no treatment or a standard treatment.[6]"[7]
Proof of concept
Def. a “short and/or incomplete realization of a certain method or idea to demonstrate its feasibility"[8] is called a proof of concept.
Def. evidence that demonstrates that a concept is possible is called proof of concept.
The proof-of-concept structure consists of
- background,
- procedures,
- findings, and
- interpretation.[9]
See also
References
- 1 2 3 K. J. McCarthy, A. Baciero, B. Zurro, and TJ-II Team (June 12-16 2000). Impurity Behaviour Studies in the TJ-II Stellarator, In: 27th EPS Conference on Contr. Fusion and Plasma Phys.. 24B. Budapest: ECA. pp. 1244-7. http://crpppc42.epfl.ch/Buda/pdf/p3_116.pdf. Retrieved 2013-01-20.
- ↑ D. R. Bates (October 1978). "Forbidden oxygen and nitrogen lines in the nightglow". Planetary and Space Science 26 (10): 897-912. doi:10.1016/0032-0633(78)90073-9. http://www.sciencedirect.com/science/article/pii/0032063378900739. Retrieved 2013-01-16.
- ↑ C. B. Collins, J. M. Carroll, K. N. Taylor, and F. W. Lee (October 1, 1978). "A regenerative power amplifier operating on the blue-green line of the nitrogen ion laser". Applied Physics Letters 33 (7): 624-6. doi:10.1063/1.90484.
- ↑ "nitrogen, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. September 22, 2013. Retrieved 2013-10-05.
- ↑ Klaus Hinkelmann, Oscar Kempthorne (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9. http://books.google.com/?id=T3wWj2kVYZgC&printsec=frontcover.
- ↑ R. A. Bailey (2008). Design of comparative experiments. Cambridge University Press. ISBN 978-0-521-68357-9. http://www.cambridge.org/uk/catalogue/catalogue.asp?isbn=9780521683579.
- ↑ "Treatment and control groups, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 18, 2012. Retrieved 2012-05-31.
- ↑ "proof of concept, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. November 10, 2012. Retrieved 2013-01-13.
- ↑ Ginger Lehrman and Ian B Hogue, Sarah Palmer, Cheryl Jennings, Celsa A Spina, Ann Wiegand, Alan L Landay, Robert W Coombs, Douglas D Richman, John W Mellors, John M Coffin, Ronald J Bosch, David M Margolis (August 13, 2005). "Depletion of latent HIV-1 infection in vivo: a proof-of-concept study". Lancet 366 (9485): 549-55. doi:10.1016/S0140-6736(05)67098-5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1894952/. Retrieved 2012-05-09.
External links
| |||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a chemistry resource . |