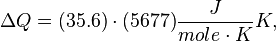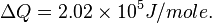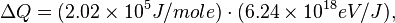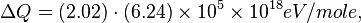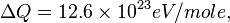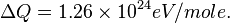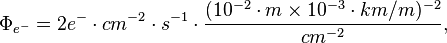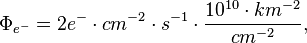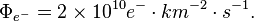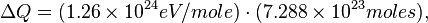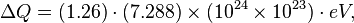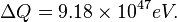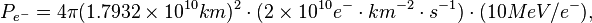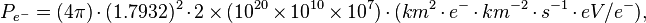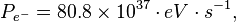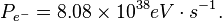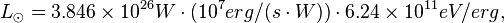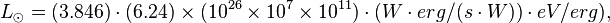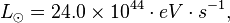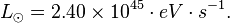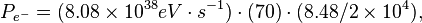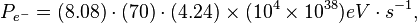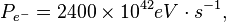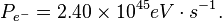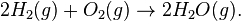Chemicals/Hydrogens
< ChemicalsHydrogens is a lecture on the general nature and specific characteristics of various natural and hominin-made hydrogens. It is an offering from the school of chemistry.
Theoretical hydrogens
Def. any "specific [...] element [such as hydrogen]"[1] is called a hydrogen.
Emissions
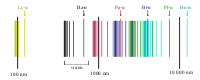
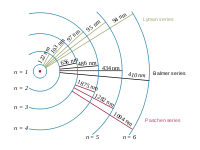
"The emission spectrum of atomic hydrogen is divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to electrons moving between energy levels in the atom. The spectral series are important in astronomy for detecting the presence of hydrogen and calculating red shifts. ... [T]he spectral lines of hydrogen correspond to particular jumps of the electron between energy levels. The simplest model of the hydrogen atom is given by the Bohr model. When an electron jumps from a higher energy to a lower, a photon of a specific wavelength is emitted."[2]
"The spectral lines are grouped into series according to n'. Lines are named sequentially starting from the longest wavelength/lowest frequency of the series, using Greek letters within each series. For example, the 2 → 1 line is called "Lyman-alpha" (Ly-α), while the 7 → 3 line is called "Paschen-delta" (Pa-δ). Some hydrogen spectral lines fall outside these series, such as the 21 cm line; these correspond to much rarer atomic events such as hyperfine transitions.[3] The fine structure also results in single spectral lines appearing as two or more closely grouped thinner lines, due to relativistic corrections.[4]"[2].
"The energy differences between levels in the Bohr model, and hence the wavelengths of emitted/absorbed photons, is given by the Rydberg formula[5]:
where n is the initial energy level, n′ is the final energy level, and R is the Rydberg constant. Meaningful values are returned only when n is greater than n′ and the limit of one over infinity is taken to be zero."[2]
"The familiar red H-alpha [Hα 656 nm] spectral line of hydrogen gas, which is the transition from the shell n = 3 to the Balmer series shell n = 2, is one of the conspicuous colors of the universe. It contributes a bright red line to the spectra of emission or ionization nebula, like the Orion Nebula, which are often H II regions found in star forming regions. In true-color pictures, these nebula have a distinctly pink color from the combination of visible Balmer lines that hydrogen emits."[6]
"A hydrogen-alpha filter is an optical filter designed to transmit a narrow bandwidth of light generally centered on the H-alpha wavelength. They are characterized by a bandpass width that measures the width of the wavelength band that is transmitted.[7] These filters are manufactured by multiple (~50) layers of vacuum-deposited layers. These layers are selected to produce interference effects that filter out any wavelengths except at the requisite band.[8] Alternatively, an etalon may be used as the narrow band filter (in conjunction with a "blocking filter" or energy rejection filter) to pass only a narrow (<0.1 nm) range of wavelengths of light centred around the H-alpha emission line. The physics of the etalon and the dichroic interference filters are essentially the same (relying on constructive/destructive interference of light reflecting between surfaces), but the implementation is different (an interference filter relies on the interference of internal reflections). Due to the high velocities sometimes associated with features visible in H-alpha light (such as fast moving prominences and ejections), solar H-alpha etalons can often be tuned (by tilting or changing the temperature) to cope with the associated Doppler effect."[9]
The Balmer series of emission lines from hydrogen occur in the visible spectrum of the Sun at: 397, 410, 434, 486, and 656 nm.
Hydrogen has two emission lines that occur in an electron cyclotron resonance (ECR) heated plasmas at 397.007 nm of the Balmer series (Hε) and 434.05 nm Hγ.[10]
Subatomics
Def. the "lightest and most common isotope of hydrogen, having a single proton and no neutrons- 11H"[11] is called protium.
Def. an "isotope of hydrogen formed of one proton and one neutron in each atom - 21H"[12] is called deuterium.
"Heavy water is “heavy” because it contains deuterium."[12]
"There were about 80 deuteriums for every million protiums, and virtually no tritium."[12]
Def. a "radioactive isotope of the element hydrogen, (symbol T or 31H), having one proton and two neutrons"[13] is called tritium.
Def. a "highly unstable, synthetic isotope of the element hydrogen, 41H, having one proton and three neutrons"[14] is called quadrium.
11H(p,β+ν)21H
At "10-million-kelvin ..., hydrogen fuses to form helium in the proton-proton chain reaction:[15]
- 411H → 221H + 2e+ + 2νe (4.0 MeV + 1.0 MeV)
- 211H + 221H → 232He + 2γ (5.5 MeV)
- 232He → 42He + 211H (12.9 MeV)"[16]
"These reactions result in the overall reaction:
- 411H → 42He + 2e+ + 2γ + 2νe (26.7 MeV)
where e+ is a positron, γ is a gamma ray photon, νe is a neutrino, and H and He are isotopes of hydrogen and helium, respectively. The energy released by this reaction is in millions of electron volts, which is actually only a tiny amount of energy."[16]
"The light elements deuterium, lithium, beryllium, and boron pose a special problem for any theory of the origin of the elements which proposes that all the elements are built up from hydrogen in the stars. ... The difficulty arises because the lifetimes of these elements against proton capture, at the temperatures and pressures at which most stellar matter exists, are short compared to the stable lifetimes of stars. These elements then cannot be produced in stellar interiors unless they are transported rapidly to the surface, and if they are produced at the surface, non-equilibrium processes must be involved. Further, they can exist in significant quantities at the surface only in the absence of rapid mixing to the interior."[17]
Gases
Hydrogen can exist as a diatomic gas (H2) or a monatomic gas. Each hydrogen isotope (hydrogen, deuterium, or tritium) can exist in each form of the gas.
Diatomic gases


Molecular hydrogen gas is excited in the discharge tube shown on the right. When an electron returns to a lower energy orbital state the purple color is observed.
"Molecular hydrogen (H2) [is] a colourless, odourless and flammable gas at room temperature."[18]
"The familiar red H-alpha [Hα 656 nm] spectral line of hydrogen gas, which is the transition from the shell n = 3 to the Balmer series shell n = 2, is one of the conspicuous colors of the universe. It contributes a bright red line to the spectra of emission or ionization nebula, like the Orion Nebula, which are often H II regions found in star forming regions. In true-color pictures, these nebula have a distinctly pink color from the combination of visible Balmer lines that hydrogen emits."[6]
A "high-resolution spectrum of the Becklin-Neugebauer (BN) infrared point source located in [the region of the Orion Nebula] ... with the Steward Observatory 2.29 m (90 inch) telescope ... [confirmed] the reality of [the 2.12 μ] line ... on 1976 January 15 and 16. The line was then identified by R. Treffers as the S(1) line of the 1-0 vibration-rotation quadrupole spectrum of H2. Six other lines of the same band were also found. The presence of two of our lines has been confirmed by Grasdalen and Joyce (1976). Electronic transitions of interstellar H2 have previously been observed in the ultraviolet (Carruthers 1970; Smith 1973; Spitzer et al. 1973)."[19]
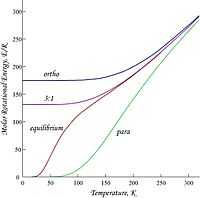
Diatomic hydrogen gas apparently exists in two distinct forms which can mix: orthohydrogen and parahydrogen. On the right is a graph of molar energies of orthohydrogen and parahydrogen, plus an equilibrium mixture obtained when a catalyst is present to allow for ortho-para interconversion. The curve marked 3:1 is the ortho:para ratio at room temperature that will persist if no catalyst is present during cooling. Orthohydrogen has the spins of the two protons parallel while para hydrogen has them antiparallel.
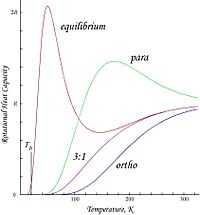
The second graph shows the molar heat capacities for the same gases versus temperature.
Monatomic gases
As temperature increases in an astronomical object composed of H2, the molecules begin to dissociate.
"At a temperature of 8000 K, hydrogen gas is 99.99 percent monatomic."[20]
where 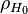 is an initial concentration [H] at low temperatures as partial particle density,
is an initial concentration [H] at low temperatures as partial particle density, 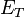 is the dissociation energy 4.52 eV, k is Boltzmann's contant (8.6173324(78)×10−5 eV K-1), and T is temperature in K.
is the dissociation energy 4.52 eV, k is Boltzmann's contant (8.6173324(78)×10−5 eV K-1), and T is temperature in K.
Using
- what is the concentration of H ([H]) at T = 8000 K?
- what is [H] at T = 800 K?
- at what temperature is [H] = 1?
- what is [H] at T = 5778 K?
Liquids

The image on the right shows that liquid hydrogen can be poured.
Solids
"Slush hydrogen [is] a mixture of solid and liquid hydrogen".[21]
Solid hydrogen is achieved by decreasing the temperature below hydrogen's melting point of 14.01 K.[22]
Bio-organics
"Use of an appropriate hydrogen level is necessary to favor dehalogenation of chlorinated solvents, such as tetrachloroethene (PCE) and trichloroethene (TCE), over other hydrogen using processes. ... the competition between dehalogenators and other microorganisms occurring in a benzoate-acclimated dehalogenating methanogenic mixed culture. ... the dehalogenators competed best against methanogens and homoacetogens when the hydrogen level was maintained between 2 and 11 nM."[23]
"In contrast, with hydrogen as the primary electron donor, homoacetogens became the dominant group in hydrogen utilization with their advantageous kinetic properties."[23]
Sun
Depending primarily upon gas temperature, the presence of gas may be used to determine the composition of the gas object observed, at least the outer layer. Early spectroscopy[24] of the Sun using estimates of "the line intensities of several lines by eye [to derive] the abundances of ... elements ... [concluded] that the Sun [is] largely made of hydrogen."[25]
The composition in the photosphere of the modern-day Sun, by mass, is 74.9% hydrogen and 23.8% helium.[26]
The visible light we see is produced as electrons react with hydrogen atoms to produce H− ions.[27][28]
Photosphere volume
R⊙eq ≈ 6.955 x 105 km. The thickness of the photosphere is about 400 km. R⊙p ≈ 6.951 x 105 km.
Photosphere hydrogen
The density of the Sun is about 2 x 10-4 kg m-3. Or,
One mole of H2 (gas) has a mass of 2.016 x 10-3 kg. The molar density of the photosphere may be
Constant volume specific heat capacity
For H2 (gas) the molar constant-volume heat capacity at 298 K is 20.18 J/(mol · K). At 2000 K it is about 25 J/(mol · K). Using a linear extrapolation,
for 5777 K, yields
Before calculating the amount of energy or power necessary to heat the coronal clouds around the Sun, let's see if the influx of electrons from outside the heliosphere may be able to heat the surface of the photosphere (p) to 5777 K from 100 K.
Photosphere heating
Voyager 1 is 17,932,000,000 km (119.9 AU) from the Sun at RA 17.163h Dec +12.44°, ecliptic latitude of 34.9°.
For this laboratory example, let the electron flux be 2 e- cm-2 s-1 diffusing into our solar system from elsewhere in the galaxy. Each of these electrons has an energy of 10 MeV.
If the electron flux measured by Voyager 1 is close to 2 e- cm-2 s-1 where each electron averages 10 MeV and these electrons are heading for the Sun, then each electron may strike the photosphere from anywhere in a sphere around the Sun.
To heat the photosphere to 5777 K takes
The power (P) that may be deposited on the photospheric surface of the Sun is
The luminosity (in Watts, W) of the Sun is 3.846 x 1026 W. In eV/s this is
If the energy of the incoming electrons is 700 MeV and the flux is 8.48 x 104 e- cm-2 s-1, then the power from the incoming electrons would be
Milky Way

"Spectra of the helium 2.06 µm and hydrogen 2.17 µm lines ... confirm the existence of an extended region of high-velocity redshifted line emission centered near [Sgr A*/IRS 16]."[29]
Technology


The first image on the left shows a liquid hydrogen bubble chamber used to detect subatomic particles from a bevatron.
On the right, the second image down, a Space Shuttle Main Engine burns diatomic hydrogen from the liquid hydrogen fuel tank with diatomic oxygen from the liquid oxygen fuel tank, producing a nearly invisible flame at full thrust.
Chemically,
See also
References
- ↑ "chemical, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 9 March 2015. Retrieved 2015-07-16.
- 1 2 3 "Hydrogen spectral series, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 2, 2012. Retrieved 2012-05-14.
- ↑ "The Hydrogen 21-cm Line". Hyperphysics. Georgia State University. 2004-10-30. Retrieved 2009-03-18.
- ↑ Richard L. Liboff (2002). Introductory Quantum Mechanics. Addison-Wesley. ISBN 0-8053-8714-5.
- ↑ Niels Bohr (1985), "Rydberg's discovery of the spectral laws", in J. Kalckar, N. Bohr: Collected Works, 10, North-Holland Publ.
- 1 2 "Balmer series, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 2, 2012. Retrieved 2012-07-11.
- ↑ "Filters". Astro-Tom.com. Retrieved 2006-12-09.
- ↑ D. B. Murphy, K. R. Spring, M. J. Parry-Hill, I. D. Johnson, M. W. Davidson. "Interference Filters". Olympus. Retrieved 2006-12-09.
- ↑ "H-alpha, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. December 30, 2011. Retrieved 2012-07-11.
- ↑ K. J. McCarthy, A. Baciero, B. Zurro, and TJ-II Team (June 12-16 2000). Impurity Behaviour Studies in the TJ-II Stellarator, In: 27th EPS Conference on Contr. Fusion and Plasma Phys.. 24B. Budapest: ECA. pp. 1244-7. http://crpppc42.epfl.ch/Buda/pdf/p3_116.pdf. Retrieved 2013-01-20.
- ↑ SemperBlotto (12 November 2005). "protium, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-07-20.
- 1 2 3 "deuterium, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 16 July 2015. Retrieved 2015-07-20.
- ↑ "tritium, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. 16 July 2015. Retrieved 2015-07-20.
- ↑ SemperBlotto (2 June 2012). "quadrium, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2015-07-20.
- ↑ G. Wallerstein, I. Iben Jr., P. Parker, A. M. Boesgaard, G. M. Hale, A. E. Champagne, C. A. Barnes, F. KM-dppeler, V. V. Smith, R. D. Hoffman, F. X. Timmes, C. Sneden, R. N. Boyd, B. S. Meyer, D. L. Lambert (1999). "Synthesis of the elements in stars: forty years of progress". Reviews of Modern Physics 69 (4): 995–1084. doi:10.1103/RevModPhys.69.995. http://authors.library.caltech.edu/10255/1/WALrmp97.pdf. Retrieved 2006-08-04.
- 1 2 "Star, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 13, 2013. Retrieved 2013-07-12.
- ↑ Walter K. Bonsack (November 1959). "The Abundance of Lithium and Convective Mixing in Stars of Type K". The Astrophysical Journal 130 (11): 843-71. doi:10.1086/146777.
- ↑ "hydrogen, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. September 1, 2013. Retrieved 2013-10-05.
- ↑ T. N. Gautier II and Uwe Fink, Richard R. Treffers, and Harold P. Larson (July 15, 1976). "Detection of Molecular Hydrogen Quadrupole Emission in the Orion Nebula". The Astrophysical Journal 207 (07): L129-33. doi:10.1086/182195. http://adsabs.harvard.edu/full/1976ApJ...207L.129G. Retrieved 2013-10-05.
- ↑ Paul A. Tipler, Gene Mosca (May 1, 2007). Physics for Scientists and Engineers. Macmillan. pp. 1172. ISBN 142920124X. http://books.google.com/books?id=AttDBYgLeZkC&pg=PA614&lpg=PA614&source=bl&ots=mDyTt5SY03&sig=-IdefwfOk591NKKnKm2iPsorRxo&hl=en&sa=X&ei=XkPGUs6UCdDqkAeeuYF4&ved=0CDAQ6AEwBA. Retrieved 2014-01-02.
- ↑ Nancy B. McNelis, Terry L. Hardy, Margaret V. Whalen, Maureen T. Kudlac, Matthew E. Moran, Thomas M. Tomsik and Mark S. Haberbusch (3-7 April 1995). "A Summary of the Slush Hydrogen Technology Program for the National Aero-Space Plane". Lewis Research Center, Cleveland, Ohio 44135-3191 USA: National Aeronautics and Space Administration. Retrieved 2015-07-20.
- ↑ James Dewar (1899). "Sur la solidification de l'hydrogène". Annales de Chimie et de Physique 18: 145–150. http://gallica.bnf.fr/ark:/12148/bpt6k349183/f143.table.
- 1 2 Yanru Yang and Perry L. McCarty (November 15, 1998). "Competition for Hydrogen within a Chlorinated Solvent Dehalogenating Anaerobic Mixed Culture". Environmental Science & Technology 32 (22): 3591-7. doi:10.1021/es980363n. http://pubs.acs.org/doi/abs/10.1021/es980363n. Retrieved 2013-08-28.
- ↑ H. N. Russell (1929). The Astrophysical Journal 70: 11-82.
- ↑ Sarbani Basu and H. M. Antia (March 2008). "HelioseismologyandSolarAbundances". Physics Reports 457 (5-6): 217-83. doi:10.1016/j.physrep.2007.12.002.
- ↑ Lodders, K. (2003). "Abundances and Condensation Temperatures of the Elements". Meteoritics & Planetary Science 38 (suppl.): 5272. doi:10.1086/375492. http://www.lpi.usra.edu/meetings/metsoc2003/pdf/5272.pdf.
- ↑ E.G. Gibson (1973). The Quiet Sun. NASA.
- ↑ Shu, F.H. (1991). The Physics of Astrophysics. 1. University Science Books. ISBN 0-935702-64-4.
- ↑ T. R. Geballe, K. Krisciunas, J. A. Bailey, and R. Wade (April 1, 1991). "Mapping of infrared helium and hydrogen line profiles in the central few arcseconds of the Galaxy". The Astrophysical Journal 370 (4): L73-6. doi:10.1086/185980. http://adsabs.harvard.edu/abs/1991ApJ...370L..73G. Retrieved 2012-08-03.
External links
| |||||||||||||||||||||||||||||||||||
![]() This is a research project at http://en.wikiversity.org
This is a research project at http://en.wikiversity.org
| |
Development status: this resource is experimental in nature. |
| |
Educational level: this is a research resource. |
| |
Resource type: this resource is an article. |
| |
Resource type: this resource contains a lecture or lecture notes. |
| |
Subject classification: this is a Geology resource. |
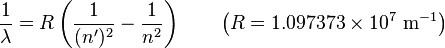
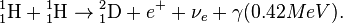
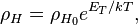
![[H] = 70400 e^{-4.52/(0.00008617T)}](../I/m/b1efa97b8375510ccbe2ee311067e11b.png)
![V_{\odot p} = \frac{4\pi}{3} [R_{\odot eq}^3 - R_{\odot p}^3] km^3,](../I/m/7fc7372bdb96b3d3ed282fcbbf6c6c81.png)
![V_{\odot p} = \frac{4\pi}{3} [6.955^3 - 6.951^3] \times 10^{15} km^3,](../I/m/958cbed14c983c446f5da856c174997a.png)
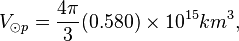
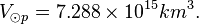
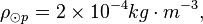
![\rho_{\odot p} = 2 \times 10^{-4} kg \cdot [10^{-3} km]^{-3},](../I/m/2c3fc1b5d5a035339a7cadb721096144.png)
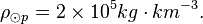
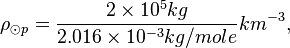
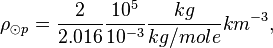
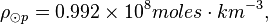
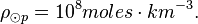
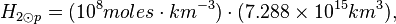
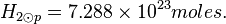
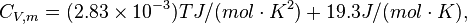
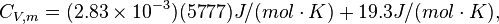
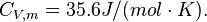
![\Delta Q = [35.6 J/(mol \cdot K)] \cdot (5777 - 100) K,](../I/m/44c0cfd75512346b741fab125e120873.png)