Bonding and chemical structure
| | Subject classification: this is a chemistry resource . |
Chemical Bonding
The force that holds two or more atoms together is called a chemical bond. The process by which bonds are formed is called chemical bonding. When a bond is formed, a chemical reaction takes place. The result of a reaction is the formation of two or more substances.
Valence electrons
- The highest principal shell of an atom, in which there is at least one electron, is called the valence shell.
- The electrons on the valence shell are called valence electrons.
- Main group elements can hold a maximum of eight electrons in their valence shell. A full valence shell is known as an "octet".
Chemical bonds are formed by sharing or transferring electrons. In general, most main group elements react in order to achieve a full valence shell. Elements in Group 18 (sometimes referred to as the noble gases) will usually not react, since their valence shell is already full.
Ionic bonding
Ionic (or electrovalent) bonding is the process by which electrons can transfer between valence shells of atoms. Since electrons are negatively charged, the loss or gain of an electron leads to charged atoms, called "ions".
- A cation is a positively charged ion. It is formed when an atom of neutral charge loses one or more electrons.
- An anion is a negatively charged ion. It is formed when an atom of neutral charge gains one or more electrons.
- The amount of energy that is needed to remove the most loosely bound electron from a neutral atom in the gas phase is called the First Ionization Energy. The amount of energy needed to remove the second electron is called the Second Ionization Energy. The amount of energy needed to remove the third electron is called the Third Ionization Energy.
Covalent bonding
One rule for predicting when an ionic bond will take place is when the difference between electronegativities is 1.7 or greater. In other cases atoms bond by sharing electrons on the valence shells. The force that holds two atoms together in this case is called a covalent bond. A covalent bond can be described as the mutual attraction that positive nuclei have for the shared, negatively charged bonding electrons. The process, by which electrons are sharing is called covalent bonding.
- The substances that are held by covalent bonds are molecular substances.
- Substances that are held by ionic bonds are ionic substances.
The group of atoms held together by covalent bonds is called a molecule.
Diatomic molecules
- Molecules that consist of two atoms shared covalently, such us Cl2, H2 (1e), F2, O2, N2, etc.
- Covalent bonding may be presented in three ways:
- 1. Nonpolar. The difference between electronegativities equal 0.
- 2. Polar. The difference between electronegativities not equal 0.
- 3. Coordinate. When a lone pair of electrons is shared.
Ionic substances
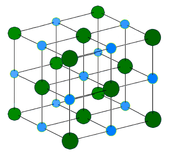
- 1. Have a high melting point.
- 2. Good conductors of heat and electricity when melted or dissolved.
- 3. Ionic substances readily dissolve in water to form neutral solutions.
Molecular substances
- 1. They are soft.
- 2. They are poor conductors of heat and electricity.
- 3. They have a low melting point.
Examples: H2O, NH3, C6H12O6.
Network solids
- 1. They are hard.
- 2. Poor conductors of heat and electricity.
- 3. Good insulators.
- 4. Very high melting point.
Examples: diamond, graphite, SiC, SiO2. (They have 4 valence electrons). i