Structural Biochemistry/Organic Chemistry/Organic Functional Group/Carboxyl
< Structural Biochemistry < Organic Chemistry < Organic Functional GroupIntroduction
A carboxyl group consists of a carbon double-bonded to an oxygen and also bonded to a -OH group. Compounds with carboxyl groups are called carboxylic acids or organic acids. The carboxyl group can act as an acid when by donating a proton (H+) to a solution and becoming ionized. Under biological conditions at pH~7, carboxyl groups are usually deprotonated, meaning they lose a H+, and become negatively charged. An example of a carboxyl group in the body would be carbonic acid, formed from the hydration of a carbon dioxide. Under biological conditions, carbonic acid usually dissociates into bicarbonate ion.
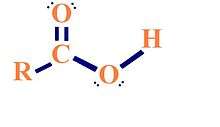
Properties
Carboxyl groups have an electronegative oxygen atom double bonded to a carbon atom. This carbon-oxygen bond is very polar and the fact that its a double bond increases the polarity of the bond. As a result of the polarity, compounds containing carboxyl groups usually have higher melting points, boiling points and have hydrophilic centers. Moreover the higher melting point and higher boiling point can be attributed to the fact that they can form hydrogen bonds both in the liquid and solid state. Fatty acids are examples of compounds that have hydrophilic centers due to their carboxyl groups. Also, carboxyl groups, especially when present in molecules with a low molecular weight tend to be highly volatile and therefore tend to have strong odors. The pKa of carboxyl groups usually range from 4-5.
Nomenclature
In naming organic molecules with multiple functional groups, the carboxyl group takes precedence in naming over any other functional group. Therefore when naming a molecule such as an alkane that contains a carboxyl group, the -e on the alkane is replaced by -oic acid. Also, when numbering the chain of the organic molecule that contains a carboxyl group, the carboxy carbon is labeled as the number 1 carbon. Molecules with two carboxyl groups would use instead the -dioic suffix.
Bonding
The polarity of the carbon-oxygen bond makes the carbon very susceptible to nucleophilic attack. Upon attack, the electrons of the double bond will migrate to the oxygen atom in order to maintain the octet for the carbon atom. The oxygen will now be negatively charged and a tetrahedral intermediate has been formed. The double bond will reform when the migrated electrons on the oxygen atom move back into the double bond to oxygen while the carbonyl carbon attacked expels the -OH group as a leaving group. While the expulsion of an -OH group is energetic unfavorable, the formation of the energetically favorable carbon-oxygen double bond helps overcomes this obstacle. Other ways to overcome this obstacle is to convert the -OH group into a better leaving group. The polarity giving the oxygen a partially negative charge also makes the carboxylic acid susceptible to electrophilic attack. An example of this is the hydrolysis of a carboxylic acid under acidic conditions where a proton acts as an electrophile and attacks at the oxygen which is doubly bonded to the carbon.
Amino Acids
The carboxyl group is a major component of amino acids. The carboxyl group, along with the amino group, allows amino acids to be zwitterions where both the amino group and the carboxyl group are charged. Since the carboxyl group can be deprotonated, it can impart a negative charge onto the amino acid. The carboxyl group is also key in the formation of peptide bonds. The carboxyl group of an amino acid can be attacked by the amino group of another amino acid. The nitrogen group of the amino group acts as the nucleophile and attacks the carbon of the carboxyl group. Carboxyl groups are also present on the side chains of two amino acids, Aspartate and Glutamate. These amino acids allow for hydrogen bonding and the formation of salt bridges, which help stabilize the structure of proteins.
From Nature
- Formic acid (HCOOH), the simplest carboxylic acid with only one carboxyl group, is primarily responsible for the pain caused by insects' bites(mostly Hymenoptera, like bees and ants).
- Acetic acid (CH3COOH) can be biologically synthesized by either aerobic or anaerobic fermentation, a process used to make vinegar. The aerobic process requires warm ethanol (CH3CH2OH) and oxygen with Acetobacter. The anearobic process requires only sugar(C6H12O6) as input chemical, and acetogen can then give carboxylic acids as output. It should be noted that aerobic process is still dominantly applied, because acetogens used for anaerobic processes show less tolerance to acidic environments. In other words, acetogens will be killed if too much acid is produced.
- Propanoic acid (CH3CH2COOH,or C2H5COOH). It can be formed by breakdown of fatty acids with odd numbers of carbon atoms. During such metabolic process, propanoic acid undergoes condensation reaction with the thiol end of CoA (coenzyme A) to form propionyl-CoA. Propanoic acid can also be synthesized by anaerobic respiration of bacteria called propionybacterium.
- Butanoic Acid (C3H7COOH) can be found in some naturally occurring esters, such as hexyl butanoate from oil of Heracleum and octyl butanoate in parsnip. The fermentation (biological) method of butanoic acid production was discovered by Louis Pasteur in 1861. The overall process takes one mole of glucose and the products are one mole of butanoic acid, two moles of carbon dioxide, two moles of hygrogen gas and three moles of ATP. During such process, glucose is cleaved into two pyruvate molecules first. The pyruvate is then oxidized to acetyl CoA releasing carbon dioxide and hydrogen as by-products. The ATP is released after the acetyl CoA undergoes various enzyme reactions.
- Benzoic acid (C6H5COOH) is the main component of benzoin resin. However, it will be fairly pricey to extract benzoic acid directly from benzoin resins. Therefore most benzoic acid in the market is manufactured industrially.
- Citric acid (HOOCCH2-COH(COOH)-CH2COOH).The simple structure is similar to glycerol, which is also a biologically abundant molecule in fat. Citric acid is a well known organic acid in variety of citrus fruit such as lemon and lime. It is the essential material for citric acid cycle, a very important metabolic process. It is also very useful for modern pharmaceutical, cosmetics and other industries that include chemical processes. The industrial production of citric acid experienced a transformation from juice extraction to biosynthesis. Industrial citric acid production began in 1890 using extractions from Italian citrus exports, following the first crystallization of citric acid by Swedish chemist Carl Wilhelm Scheele in 1784. The biological methods of production was discovered in 1893 using Penicillium mold and sugar, but such process was not popular until World War I cut the Italian citrus exports. A more effecient biological production was then discovered by American food chemist James Currie by using cheap sugary mixture and mold called A. niger. Such industrial process has been used by major pharmaceutical companies like Pfizer. In addition, the extracted or biologically synthesized citric acid is precipitated by calcium hydroxide for isolation and acid is converted back from precipitate at the end.
- Oxalic acid (HOOC-COOH) is found in kidney stones as calcium oxalate, and it leads to kidney failure. It is therefore risky to eat carambola (commonly known as starfruit) and monstera due to their high oxalate content. Oxalate is also included in citric acid cycle.