Structural Biochemistry/Carbohydrates/Disaccharides
< Structural Biochemistry < CarbohydratesIn other words, disaccharies are composed by 2 sugar molecules. It is called polysaccharides.
General information
Disaccharides, the simplest polysaccharides, are the products of a condensation reaction between two monosaccharides. Disaccharide is one of four groups of Carbohydrates (monosaccharide, disaccharide, polysaccharide, and oligosaccharide).
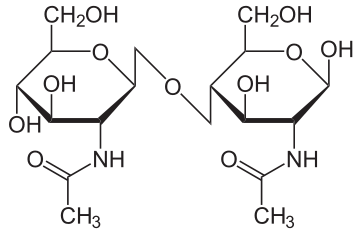
Formation
Disaccharides are formed when two monosaccharides join together by the dehydration synthesis reaction resulting in a glycosidic bond between the two monosaccharide molecules. The reaction produces water as a side product. The glycosidic bond in the picture below is a α-glycosidic bond because the bond is formed on the side opposite of the -CH2OH group.
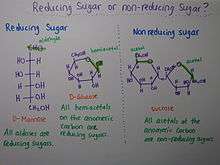
Reducing Sugars
Most disaccharides are hemiacetals. Hemiacetals contain a free aldehyde to be oxidized into carboxylic acid. These are classified as reducing sugar. For example: maltose, lactose.
Carbohydrates that are acetals are not oxidized because both of its anomeric carbon atoms are fixed in a glycosidic bond. These are classified as non-reducing sugar. For example: Sucrose.
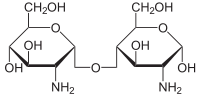
Classification
There are two basic types of disaccharides: reducing disaccharides, in which disaccharides are Hemiacetals and contain a reactive carbonyl group, they are readily oxidized to diverse products.
Non-reducing disaccharides, in which the sugar is an acetals (or ketals) that cannot readily oxidized because both anomeric carbon atoms are fixed in a glycosidic linkage in which the components bond through their anomeric centers.
Properties
The bond can form between hydroxyl groups on the two monosaccharides. Due to the different hydroxyl groups that bond, along with the alpha(α) or beta(β) position of the anomeric carbon, there are resulting disaccharides that are diastereomers differing in chemical and physical properties, depending on the monosaccharide components The α-glucoside is more stable then β-glucoside due to anomeric effect. The C-R bond has a δ* antibonding orbital. If the C-R bond is in axial position, the antibonding δ* overlaps with one of the orbital of the oxygen, which stabilize the molecule. If the C-R bond is in equatorial position, there is no overlap between orbitals making the β-glucoside less stable than the α-glucoside.
Common disaccharides
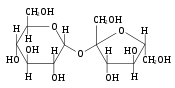
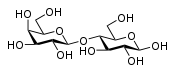
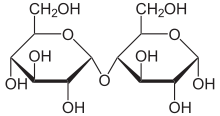
The most common disaccharides are Sucrose, Lactose, and Maltose.
Sucrose is the sugar often found in the grocery store and is produced by plants. It is a sugar derived from fructose and glucose. It is obtained from cane as a transport form of carbohydrates.
Lactose, found in milk, is formed by connecting β-D-galactose and α-D-glucose with a β-1,4-glycosidic bond.
Maltose is created by condensation reaction of the two glucoses, forming a α-1,4-O-glycosidic linkage. It is the second member of an important biochemical series of glucose chains. Maltose can be broken down into two glucose molecules by hydrolysis. In living organisms, the enzyme maltase can achieve this very rapidly.