Physics Exercises/Fundamental Constants
< Physics ExercisesTable of Constants
| Name | Symbol | Value | Units | Relative Uncertainty |
|---|---|---|---|---|
| Speed of light (in vacuum) |  |
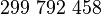 |
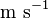 |
(exact) |
| Magnetic Constant | 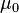 |
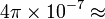 |
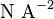 |
(exact) |
| Electric Constant | 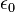 |
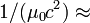 |
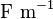 |
(exact) |
| Newtonian Gravitaional Constant | 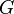 |
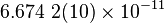 |
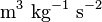 |
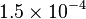 |
| Plank's Constant | 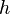 |
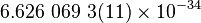 |
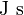 |
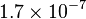 |
| Elementary charge |  |
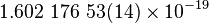 |
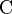 |
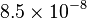 |
| Mass of the electron |  |
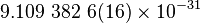 |
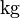 |
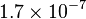 |
| Mass of the proton | 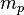 |
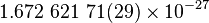 |
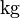 |
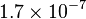 |
| Fine structure constant | 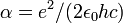 |
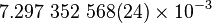 |
dimentionless | 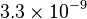 |
| Molar gass constant | 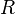 |
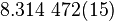 |
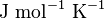 |
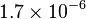 |
| Boltzman's constant | 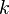 |
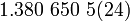 |
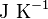 |
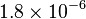 |
| Avogadro's Number | 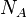 |
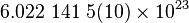 |
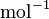 |
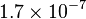 |
| Rydberg constant | 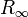 |
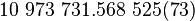 |
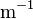 |
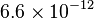 |
To Be Merged Into Table
This list is prepared in the format
- Constant (symbol) : value
- 1 Atmosphere Pressure (1 atm) : 1.0 × 105 N/m2 = 1.0 × 105 Pa
- 1 Electron Volt (1 eV) : 1.60 × 10−19 J
- Bohr radius (a0) : 5.292 × 10−11 m
- Coulomb's Law Constant (k) : 1/(4 π ε0) = 9.0 × 109 N·m2/C2
- Faraday constant (F) : 96,485 C·mol−1
- Gravitational accelaration (g) : 9.80665 m·s-2
- Mass of a neutron (mn) : 1.67495 × 10−27 kg
- Mass of Earth : 5.98 × 1024 kg
- Mass of the Moon : 7.35 × 1022 kg
- Mean radius of Earth : 6.37 × 106 m
- Mean radius of the Moon : 1.74 × 106 m
- Dirac's Constant (
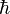 ) :
) : 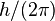 = 1.05457148 × 10−34 J·s
= 1.05457148 × 10−34 J·s - Speed of sound in air at STP : 3.31 × 102 m/s
- Unified Atomic Mass Unit (u) : 1.66 × 10−27 kg
- Vacuum Permittivity (ε0) : 8.85 × 10−12 C2/(N·m2)
| Item | Proton | Neutron | Electron |
| Mass | 1 | 1 | Negligible |
| Charge | +1 | 0 | -1 |
See Also
Wiki-links
External Links
This article is issued from Wikibooks. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.