Organic Chemistry/Introduction to reactions/Transition states
< Organic Chemistry < Introduction to reactionsTransition State
Many reactions occur in a single step when two reactant molecules collide with sufficient energy in the proper spatial orientation to create a product. Many other reactions, however, do not occur in a single step, and such reactions are said to have transition states. Multistep reactions have products that result from a series or chain of reactions, and these reactions are the kind that actually do pass through a transition state.
Intermediate Molecules
Multi-step reactions have an intermediate molecule that forms as a sort-of halfway point between the reactant and the product. This intermediate molecule cannot be isolated in solution, because it is typically of much higher energy than either the reactants or the products.
At a basic level of organic chemistry the intermediate molecule is often merely predicted or assumed, but many are also known to exist due to experimental observations in the laboratory. Inversions and other changes in molecular configuration without another, more plausible explanation are one type of proof that certain reactions pass through a transition state.
EXAMPLE: As an example of a molecular inversion, in a 1st order nucleophilic substitution (SN1 reaction) the chirality of a carbon can be "flipped" from S to R configuration (or vice versa) 50% of the time. This is due to the chiral carbon having only sp3 hybridization of its electrons in the intermediate state, which creates a carbon center that is flat and thus subject to nucleophilic attack from either the top or the bottom. If inversion occurs then it is considered to be proof that the reaction indeed is SN1 and therefore has an intermediate molecule in its transition state.
NOTE: THIS PARAGRAPH CONTAINS SEVERAL ERRORS AND NEEDS TO BE CORRECTED.
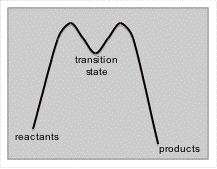
Energy Diagrams and Transition States
Observing energy diagrams of reactions, you may notice that instead of a single peak energy, the energy peaks, drops a little, peaks once again and then returns to a lower energy state than the original molecule. The "saddle" between the two peaks represents an intermediate - a place where the reaction temporarily "rests" between energy peaks. The energy peaks are defined as 'transition states' with no finite existence. Reaction intermediates will often have a finite existence. NOTE: THE DIAGRAM INCORRECTLY IDENTIFIES AN INTERMEDIATE AS A TRANSITION STATE. IN FACT, THE ENERGY PEAKS ARE TRANSITION STATES BETWEEN THE INTERMEDIATE AND EITHER THE STARTING MATERIAL OR THE PRODUCT.