Biochemistry/Electron Transport Chain
< BiochemistryThe electron transport chain is a system of molecules through which electrons are transferred to generate ATP. It has an important role in both photosynthesis and cellular respiration.
ETC in photosynthesis
In photosynthesis, when you are absorbed in photosystem 2, electrons are energized. They are transferred to the reaction center. From the reaction center, the electrons enter the electron transport chain and pass the etransport chain molecules. Then the 2 deenergized electrons are reenergized in Photosystem 1 (is second because PSII was discovered first)and they go to the NAPD+ reductase which transfers ther electrons to a coenzyme, converting it to NADPH+ The two electrons used must be replaced, so water is broken down producing 2 protons (H+) which concentrate in the thylakoid membrane, 2 electrons replaced in PSII and oxygen released as O2. Protons go down proton pumps and a concentration gradient forms as protons move from the stroma into the thylakoid space. Protons move down gradient through ATP synthase which forms ATP. Energy is stored.
ETC in respiration
In respiration, an electron transport chain is used to break the fall of electrons to oxygen into several energy-releasing steps. It slows down the releasing process of energy. There are a number of molecules that make up an electron transport chain. Most of these molecules are proteins, built into the inner membrane of mitochondria of eukaryotic cells and the plasma membrane of aerobically respiring prokaryotes. On the "top" end of the chain, where the energy is highest, electrons removed from oxidation of glucose are shuttled by NADH. On the "bottom" end, where the energy is lowest, Oxygen molecules pick up the electrons, and together with protons existing in the cellular environment, oxygen molecules form water. The total energy released from the oxidation of glucose by oxygen is huge, about 2880kJ/mol. (The overall oxidation of glucose is shown by reaction C6H12O6 (aq) + 6 O2 (g) → 6 CO2 (g) + 6 H2O). This energy can't be picked up by cells in a short amount of time. Therefore, electron transport chain applies a cascade of reactions to slowly release the energy, maximizing the usage of glucose. In the cascade, each "downhill" carrier is more electronegative than their "uphill" neighbors, so that the electrons can be passed "downhill" by oxidation. Oxygen is the last electron acceptor.
- There are four unique electron carrier complexes in the respiratory chain:
- Complex I: NADH dehydrogenase
- Composed of 42 different polypeptide chains.
- Has several Fe-S centers.
- NADH + H + FMN <---> NAD + FMNH2
- Transfers 2 electrons from NADH to Q.
- Pumps 4 protons from matrix to IMS.
- Complex II: Succinate Dehydrogense
- Transfers 2 electrons from succinate to coenzyme Q.
- Contains 4 different protein subunits:
- Subunit A: FAD, succinate binding
- Subunit B: 3 Fe-S centers
- Subunit C: integral membrane protein, Q binding
- Subunit D: does not participate in electron transfer to Q, instead reduces the frequency of some leaking electrons form reactive oxygen Species: ROS.
- Complex III: Ubiquinone Cytochrome C Oxidoreductase
- Composed of 22 subunits: heme groups and Fe-S centers
- Transfers of 2 electrons QH2 to Cytochrome C.
- Pumps 4 protons into IMS.
- Complex IV: Cytochrome C Oxidase
- Contains 13 subunits, prosthetic groups such as cytochromes and Fe-S centers.
- Transfers 2 electrons from cytochrome C to oxygen, reducing it to H2O.
- Pumps 2 protons into IMS
- Complex I: NADH dehydrogenase
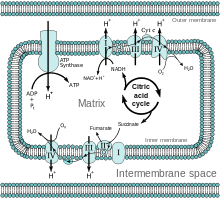
image citation:http://en.wikipedia.org/wiki/File:Mitochondrial_electron_transport_chain%E2%80%94Etc4.svg