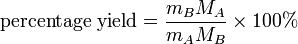A-level Chemistry/OCR (Salters)/Yield
< A-level Chemistry < OCR (Salters)Percentage yield is a useful way of saying how much of a reactant has been successfully converted to product in a chemical reaction.
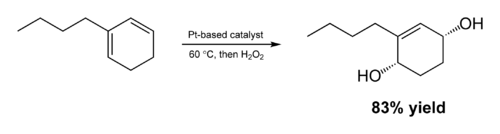
from J. Am. Chem. Soc. (2009) 131 (26), 9134–9135
If all the reactants become products, the percentage yield is 100%. If half the reactants become products and the rest become by-products or don't react at all, the percentage yield is 50%. If none of the reactants end up as products, the percentage yield is 0%.
In a nutshell
For a reaction where, ideally, one mole of starting material A is converted to one mole of products B, i.e. A→ B:
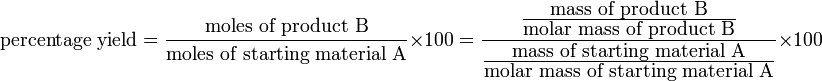
Importance
Percentage yield is important because:
- chemical reactions very often form by-products as well as the intended product
- in most reactions, not all of the reactants actually react
What is left at the end of a reaction is thus typically a mixture of product and impurities (impurities being by-products and unreacted starting materials).
Achieving high percentage yields is particularly important in industries that rely on organic synthesis, such as the pharmaceutical industry. It may require tens of steps to synthesise a drug molecule — if there are ten steps, each of which has a percentage yield of 90%, the overall yield is only 35%:
90% × 90% × 90% × 90% × 90% × 90% × 90% × 90% × 90% × 90% = (90%)10 = 35%
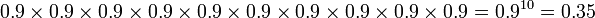
In certain reactions (such as the Haber process) reactants can be recycled, in which case high yields are less of an issue.
Percentage yield formula
There is a simple formula you can use to calculate the percentage yield of a reaction A → B:

Percentage yield effectively states the percentage of moles of reactant that become product — although this definition only applies if one mole of reactant ideally forms one mole of product, i.e. 1:1 stoichiometry.
In practice, the number of moles of A and B involved have to be calculated using the masses, m and molar masses, M, of A and B.
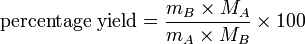
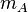 stands for the mass of substance A in g
stands for the mass of substance A in g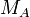 stands for the molar mass of substance A in g mol−1
stands for the molar mass of substance A in g mol−1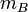 stands for the mass of substance B in g
stands for the mass of substance B in g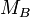 stands for the molar mass of substance B in g mol−1
stands for the molar mass of substance B in g mol−1
In words, this is:
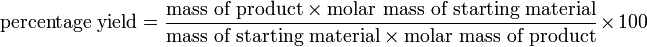
This can also be written as:

Another way of writing the same this is given below, but this form is a little untidy:
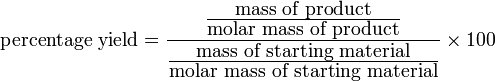
Derivation of formula
Notation
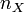 means number of moles of substance X
means number of moles of substance X
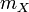 means mass of substance X
means mass of substance X
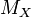 means molar mass of substance X
means molar mass of substance X
Definition of yield
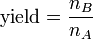
Definition of percentage yield
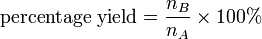
Expressing moles in terms of mass and RFM
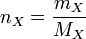 , which you may know as
, which you may know as 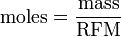
therefore, wherever 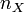 appears, it can be replaced with
appears, it can be replaced with 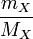
thus 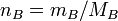
and 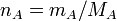
Rewriting the yield formula in terms of mass and RFM
substituting the above equations into the formula for percentage yield
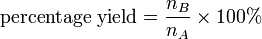
gives
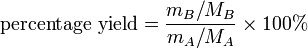
which can be rearranged to give