A-level Chemistry/OCR (Salters)/Phosphorus
< A-level Chemistry < OCR (Salters)Phosphorus is the fifteenth element in the periodic table. The phosphorus nucleus therefore contains fifteen protons.
Isotopes
Only phosphorus-31, 31P, is stable, so all naturally-occurring phosphorus is this isotope, which contains 16 neutrons. The 31P nucleus is NMR-active, and 31P NMR is widely used in chemistry.
32P is radioactive and emits beta particles; it is used in biological experiments.
Electron configuration
The electron configuration of the neutral phosphorus atom, H, in the gas phase is
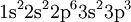
which is more concisely written as
![[\mbox{Ne}] 3\mbox{s}^2 3\mbox{p}^3 \,\!](../I/m/9c86e039455d4ea91279f52dc18bf9c5.png)
This simplifies to 2.8.5 in GCSE-style electron shell notation.
Allotropes
In its standard elemental state, phosphorus is known to exist in several infinite covalent network structures. The most important allotropes of P are:
- White phosphorus: contains discrete P4 tetrahedra (these also exist in liquid P)
- Black phosphorus: sheets of phosphorus atoms stacked on top of each other - each sheet is puckered
- Red phosphorus: amorphous, like glass
-

ball-and-stick model of a sheet of P atoms in black phosphorus
Compounds
Phosphorus forms compounds with most elements in the periodic table. Due to its larger size, phosphorus is much worse at forming double bonds than nitrogen is, so it mostly forms single bonds. One key exception is the very strong P=O bond is phosphoric acid.
Phosphates
Salts of phosphoric acid, contain the phosphate ion, PO43−.
- sodium phosphate, Na3PO4
- magnesium phosphate, Mg3(PO4)2
- aluminium phosphate, AlPO4
Phosphides
In compounds with very electropositive elements, as in sodium phosphide, Na3P, phosphorus can exist as the phosphide ion, P3−. P3− does not exist in solution because it is exceptionally reactive. For example, if you try to dissolve Na3P in water, the following reaction occurs:
- Na3P + 3H2O(l) → PH3(g) + 3NaOH(aq)
In compounds with elements whose electronegativity is similar to or greater than phosphorus, such as phosphorus trifluoride, PF3, phosphorus is covalently bonded to the other element.
Organophosphorus compounds
- Phosphines: phosphorus analogues of amines
- Organophosphates: esters of phosphoric acid
- Organophosphites: esters of phosphorous acid