A-level Chemistry/OCR (Salters)/Developing Fuels
< A-level Chemistry < OCR (Salters)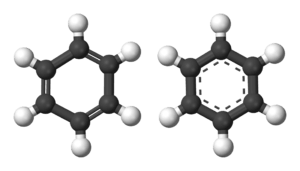
Benzene is an aromatic hydrocarbon. Instead of having three short double bonds and three long single bonds (above, left), it has six medium-length bonds (above, right).
A double bond is really two bonds, with two electrons in each bond, so four electrons in total in a double bond. Two electrons from each double bond in benzene (if the double bonds really existed, which they don't) are smeared over all six carbon atoms.
Whenever electrons are not confined to a bond between just two atoms, they are called declocalised electrons.
A double bond is really two bonds, with two electrons in each bond, so four electrons in total in a double bond. Two electrons from each double bond in benzene (if the double bonds really existed, which they don't) are smeared over all six carbon atoms.
Whenever electrons are not confined to a bond between just two atoms, they are called declocalised electrons.
Developing Fuels is the second unit in the Salters Advanced Chemistry course.
Chemical Storylines sections
- DF1: Petrol is popular
- DF2: Getting energy from fuels
- DF3: Focus on petrol
- DF4: Making petrol − getting the right octane rating
- DF5: Trouble with emissions
- DF6: Tackling the emissions problem
- DF7: Changing the fuel
- DF8: Hydrogen − a fuel for the future?
- DF9: Summary
Chemical Ideas sections
- 4.1 Energy out, energy in
- 1.3 Using equations to work out reacting masses
- 4.2 Where does the energy come from?
- 12.1 Alkanes
- 3.4 Structural isomerism
- 13.2 Alcohols and ethers
- 4.3 Entropy and the direction of change
Activities
- DF1.1 Which fuel for the future?
- DF1.2 Measuring the enthalpy change of combustion of different fuels
- DF1.3 Comparing the enthalpy changes of combustion of different alcohol
- DF2.1 Using spreadsheets to calculate enthalpy changes of combustion
- DF2.2 Making notes
- DF3.1 How do physical properties change along the alkane series?
- DF3.2 Comparing winter and summer petrol blends
- DF3.3 Auto-ignition in a test-tube
- DF4.1 Modelling and naming alkanes
- DF4.2 The octane numbers of different alkanes
- DF4.3 Using zeolites
- DF4.4 Cracking alkanes
- DF4.5 A closer look at alcohols
- DF4.6 Blend your own
- DF4.7 Why do hydrocarbons mix?
- DF4.8 Petrol - pulling it all together
- DF5 What happens to the sulphur?
- DF9 Check your notes on Developing Fuels
External links
This article is issued from Wikibooks. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.