A-level Chemistry/OCR (Salters)/Buffer solutions
< A-level Chemistry < OCR (Salters)Calculating the pH of a buffer solution
![\mbox{pH} = -\log_{10}{ \left ( K_a \frac{\left [ \mbox{acid} \right ]}{\left [ \mbox{salt} \right ]} \right ) }](../I/m/22b46a61ac99241112c10c6fd39f32ee.png)
Derivation
For any equilibrium
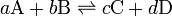
the equilibrium constant, K, is defined as
![K = \frac{ [\mbox{C}]^c [\mbox{D}]^d }{ [\mbox{A}]^a [\mbox{B}]^b }](../I/m/67c395e7d24a6c7f6b7700f8ab6074f1.png)
Therefore, for the dissociation equilibrium of any acid
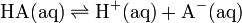
the acid dissociation constant, Ka, is defined as
![K_a = \frac{ [\mbox{H}^+ \mbox{(aq)}] [\mbox{A}^- \mbox{(aq)}] }{ [\mbox{HA} \mbox{(aq)}] }](../I/m/d250d80414d6668aa5dfcddf5f751fc7.png)
This equation can be rearranged to make [H+(aq)] the subject:
![[\mbox{H}^+ \mbox{(aq)}] = K_a \frac{ [\mbox{HA} \mbox{(aq)}] }{ [\mbox{A}^- \mbox{(aq)}] }](../I/m/7a3e84ee18508ddf2caa975621c93213.png)
Two assumptions are required:
1 Every A− ion comes from the salt
- Although this is not quite true, it is a close enough that the pH value we get from the final equation is very close to that found experimentally. It allows us to assume that
2 Every HA molecule remains undissociated
- Again, despite being slightly inaccurate, this assumption creates the following useful equation
The equations in assumptions 1 and 2 allow us to replace [A−(aq)] with [salt] and [HA(aq)] with [acid] as follows.
The effect of assumption 1 is that
![[\mbox{H}^+ \mbox{(aq)}] = K_a \frac{ [\mbox{HA} \mbox{(aq)}] }{ [\mbox{A}^- \mbox{(aq)}] }](../I/m/7a3e84ee18508ddf2caa975621c93213.png)
becomes
![[\mbox{H}^+ \mbox{(aq)}] = K_a \frac{ [\mbox{HA} \mbox{(aq)}] }{ [ \mbox{salt} ] }](../I/m/f1880fd98aa41d75ba54d0d0f49622fd.png)
The effect of assumption 2 is that
![[\mbox{H}^+ \mbox{(aq)}] = K_a \frac{ [\mbox{HA} \mbox{(aq)}] }{ [ \mbox{salt} ] }](../I/m/f1880fd98aa41d75ba54d0d0f49622fd.png)
becomes
![[\mbox{H}^+ \mbox{(aq)}] = K_a \frac{ [\mbox{acid}] }{ [ \mbox{salt} ] }](../I/m/1752809e09f727d9d1aa8b3d054fc11a.png)
By definition,
![\mbox{pH} = -\log_{10}{ \left ( \left [ \mbox{H}^+ \mbox{(aq)} \right ] \right ) }](../I/m/79fd7cc3015f662658ecdfb254599dcc.png)
so
![\mbox{pH} = -\log_{10}{ \left ( K_a \frac{\left [ \mbox{acid} \right ]}{\left [ \mbox{salt} \right ]} \right ) }](../I/m/22b46a61ac99241112c10c6fd39f32ee.png)
This article is issued from Wikibooks. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
![\left [ \mbox{HA} \mbox{(aq)} \right ] = \left [ \mbox{acid} \right ]](../I/m/0af394473035cdef32746f0cb2931c14.png)
![\left [ \mbox{A}^- \mbox{(aq)} \right ] = \left [ \mbox{salt} \right ]](../I/m/5d75b24c1a89a8874a84f415d273c891.png)