Malaria
Background
Malaria vector: Anopheles stephensi mosquito.
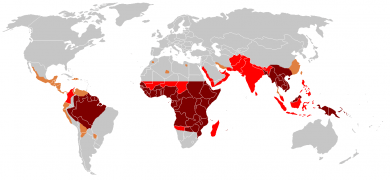
Distribution of malaria in the world (2010; see CDC website for current distribution):[1]
♦ Elevated occurrence of chloroquine- or multi-resistant malaria
♦ Occurrence of chloroquine-resistant malaria
♦ No Plasmodium falciparum or chloroquine-resistance
♦ No malaria
♦ Occurrence of chloroquine-resistant malaria
♦ No Plasmodium falciparum or chloroquine-resistance
♦ No malaria
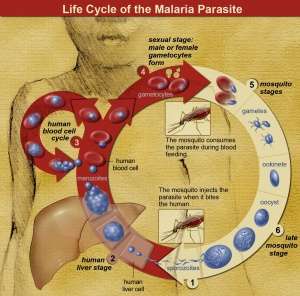
The life cycle of malaria parasites.
- Caused by parasitic protozoa species of the genus Plasmodium (P ovale, P vivax, P malariae, P knowlesi, and P falciparum) carried by the Anopheles mosquito
- P falciparum most severe
- Failure to consider for febrile illness following travel, even if seemingly temporally remote, can result in significant morbidity or mortality, especially in children and pregnant or immunocompromised patients
- Chemoprophylaxsis does not guarantee protection
- CDC Malaria Hotline: 770-488-7788
- Malaria is a US nationally notifiable disease and all cases should be reported
- Malaria vaccine with ~30% efficacy will be piloted in African countries in 2018, study to assess pediatric mortality[2]
Traveler Precautions
The CDC recommends travelers to malaria-endemic regions take the following precautions:[3]
- Chemoprophylaxis
- Use of insecticide-treated bed nets
- Use of DEET-containing insect repellents
- Wear long-sleeve shirts and pants
Clinical Features
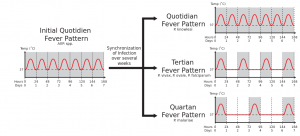
Different fever patterns observed in Plasmodium infections.

Child with malaria.
- Fever + exposure to endemic country
- Cyclic fever only after chronic infection
- Headache, cough, GI symptoms
Classification
Severe
- Any one of the following:
- Altered mental status/coma
- Severe normocytic anemia [hemoglobin < 7]
- Renal failure
- ARDS
- Hypotension
- DIC
- Spontaneous bleeding
- Acidosis
- Hemoglobinuria
- Jaundice
- Hepatomegaly
- Splenomegaly
- Repeated generalized seizures
- Parasitemia >5%
Uncomplicated
- None of the above
Differential Diagnosis
Evaluation
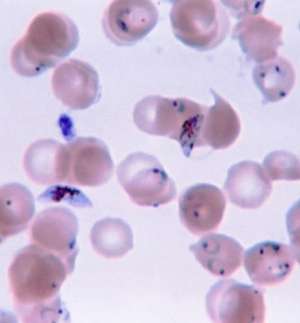
Ring-forms and gametocytes of Plasmodium falciparum in human blood
- First smear positive in >90% of cases (thick and thin Giemsa stain)
- If initial negative, must be repeated BID x 2-3 days for proper exclusion of malaria
- Determines degree of parasitemia and type (e.g. P. falciparum)
- Additional lab findings
- Normocytic anemia
- Thrombocytopenia
- ↑ ESR
- ↑ LDH
- LFT abnormalities
- ↑ Cr
- Hyponatremia
- Hypoglycemia
- False positive VDRL
Management
- Mixed infections involving more than one species of Plasmodium may occur in areas of high endemicity (have a low threshold for including treatment for P falciparum)[4]
- Hyponatremia in the setting of hypovolemia does not require treatment beyond rehydration
- Treat hypoglycemia
- Check HIV status (coinfection can lead to worse clinical outcomes)
- Exchange transfusion for patients with:
- P falciparum malaria with a parasitemia greater than 10%
- Life-threatening complications (ie, coma, respiratory failure, coagulopathy, fulminant kidney failure)
- For specific dosing see the CDC Recommendations or call the Malaria CDC Hotline(855) 856-4713
Uncomplicated Malaria
- Uncomplicated:
- No evidence of organ dysfunction
- Parasitemia <5%
- Able to tolerate PO
- Hospitalize:
- Severe clinical manifestations in non-immune host for P. falciparum or P. knowlesi
- Report to state health department
- For non-pregnant patients (3 day course)
- Artemether + lumefantrine
- Artesunate + amodiaquine
- Artesunate + mefloquine
- Dihydroartemisinin + piperaquine
- Artesunate + sulfadoxine–pyrimethamine (SP)
- For pregnant (1st trimester)
- Quinine + clindamycin x 7 days
- Additional considerations
- Avoid artesunate + SP in HIV/AIDS patients taking co-trimoxazole
- Avoid artesunate + amodiaquine in HIV/AIDS patients taking efavirenz or zidovudine
Severe Malaria
- Do not delay treatment in the unstable patient if strong suspicion for malaria as initial smear may be falsely negative
- Treatment (IV for ≥24 hours then 3 days PO course)
- Artesunate (IV)
- Clears malaria faster than quinine
- Distributed only through CDC
- Quinidine (IV) also appropriate choice; more available in US
- Artesunate (IV)
Cerebral Malaria
- Insufficient evidence for or against giving antiepileptics
- For severe cerebral edema, mannitol and steroids have not shown a demonstrable benefit
Disposition
Admit for
- Patients with suspected or confirmed P falciparum or P knowlesi infection
- Young children
- Pregnant women
- Immunocompromised patients
Admit to ICU for
- Severe complications (e.g.coagulopathy or end-organ failure)
- Cerebral malaria (e.g. altered mental status, repeated seizures, coma)
- Parasitemia
- >2% in non-immune (i.e. travelers)
- >5% in semi-immune (i.e. locals)
See Also
- Travel Medicine
- Parasitic Diseases
External Links
References
- "Malaria". US Centers for Disease Control and Prevention. April 15, 2010. Archived from the original on April 16, 2012. Retrieved 2012-05-02.
- WHO. Ghana, Kenya and Malawi to take part in WHO malaria vaccine pilot programme. 24 April 2017. http://www.afro.who.int/en/media-centre/pressreleases/item/9533-ghana-kenya-and-malawi-to-take-part-in-who-malaria-vaccine-pilot-programme.html
- WHO Malaria Policy Advisory Committee and Secretariat. Malaria Policy Advisory Committee to the WHO: conlusionsions and recommendations of September 2013 meeting. Malar J. 2013;12(1):456
- World Health Organization. Guidelines for the treatment of malaria, 3rd ed, WHO, Geneva 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/
This article is issued from
Wikem.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.